Ceramide synthase 6 impacts T-cell allogeneic response and graft-versus-host disease through regulating N-RAS/ERK pathway
- PMID: 35513703
- PMCID: PMC9256768
- DOI: 10.1038/s41375-022-01581-6
Ceramide synthase 6 impacts T-cell allogeneic response and graft-versus-host disease through regulating N-RAS/ERK pathway
Abstract
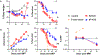
Allogeneic hematopoietic cell transplantation (allo-HCT) is an effective immunotherapy for various hematologic malignancies, predominantly through potent graft-versus-leukemia (GVL) effect. However, the mortality after allo-HCT is because of relapse of primary malignancy and followed by graft-vs-host-disease (GVHD) as a major cause of transplant-related mortality. Hence, strategies to limit GVHD while preserving the GVL effect are highly desirable. Ceramide, which serves a central role in sphingolipid metabolism, is generated by ceramide synthases (CerS1-6). In this study, we found that genetic or pharmacologic targeting of CerS6 prevented and reversed chronic GVHD (cGVHD). Furthermore, specific inhibition of CerS6 with ST1072 significantly ameliorated acute GVHD (aGVHD) while preserving the GVL effect, which differed from FTY720 that attenuated aGVHD but impaired GVL activity. At the cellular level, blockade of CerS6 restrained donor T cells from migrating into GVHD target organs and preferentially reduced activation of donor CD4 T cells. At the molecular level, CerS6 was required for optimal TCR signaling, CD3/PKCθ co-localization, and subsequent N-RAS activation and ERK signaling, especially on CD4+ T cells. The current study provides rationale and means for targeting CerS6 to control GVHD and leukemia relapse, which would enhance the efficacy of allo-HCT as an immunotherapy for hematologic malignancies in the clinic.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures

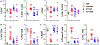
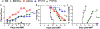

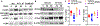

Similar articles
-
Ceramide synthesis regulates T cell activity and GVHD development.JCI Insight. 2017 May 18;2(10):e91701. doi: 10.1172/jci.insight.91701. eCollection 2017 May 18. JCI Insight. 2017. PMID: 28515365 Free PMC article.
-
Icariin protects against acute graft-versus-host disease while preserving graft-versus-leukemia activity after allogeneic hematopoietic stem cell transplantation.Phytomedicine. 2024 Sep;132:155901. doi: 10.1016/j.phymed.2024.155901. Epub 2024 Jul 18. Phytomedicine. 2024. PMID: 39067193
-
Sphingolipid metabolism in T cell responses after allogeneic hematopoietic cell transplantation.Front Immunol. 2022 Aug 16;13:904823. doi: 10.3389/fimmu.2022.904823. eCollection 2022. Front Immunol. 2022. PMID: 36052066 Free PMC article. Review.
-
Pharmacologic inhibition of PKCα and PKCθ prevents GVHD while preserving GVL activity in mice.Blood. 2013 Oct 3;122(14):2500-11. doi: 10.1182/blood-2012-12-471938. Epub 2013 Aug 1. Blood. 2013. PMID: 23908466 Free PMC article.
-
Retention of Donor T Cells in Lymphohematopoietic Tissue and Augmentation of Tissue PD-L1 Protection for Prevention of GVHD While Preserving GVL Activity.Front Immunol. 2022 May 23;13:907673. doi: 10.3389/fimmu.2022.907673. eCollection 2022. Front Immunol. 2022. PMID: 35677056 Free PMC article. Review.
Cited by
-
Mysterious sphingolipids: metabolic interrelationships at the center of pathophysiology.Front Physiol. 2024 Jan 3;14:1229108. doi: 10.3389/fphys.2023.1229108. eCollection 2023. Front Physiol. 2024. PMID: 38235387 Free PMC article. Review.
-
S1P/S1PR1 signaling differentially regulates the allogeneic response of CD4 and CD8 T cells by modulating mitochondrial fission.Cell Mol Immunol. 2022 Nov;19(11):1235-1250. doi: 10.1038/s41423-022-00921-x. Epub 2022 Sep 8. Cell Mol Immunol. 2022. PMID: 36071219 Free PMC article.
-
Ceramide signaling in immunity: a molecular perspective.Lipids Health Dis. 2025 Jul 1;24(1):225. doi: 10.1186/s12944-025-02642-2. Lipids Health Dis. 2025. PMID: 40597360 Free PMC article. Review.
-
Dimethyl fumarate ameliorates chronic graft-versus-host disease by inhibiting Tfh differentiation via Nrf2.Leukemia. 2025 Feb;39(2):473-481. doi: 10.1038/s41375-024-02475-5. Epub 2024 Nov 23. Leukemia. 2025. PMID: 39580582
-
Pretransplant Systemic Lipidomic Profiles in Allogeneic Stem Cell Transplant Recipients.Cancers (Basel). 2022 Jun 13;14(12):2910. doi: 10.3390/cancers14122910. Cancers (Basel). 2022. PMID: 35740576 Free PMC article.
References
-
- Olson MF, Marais R. Ras protein signalling. Seminars in immunology 2000. Feb; 12(1): 63–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

