Plant hormone regulation of abiotic stress responses
- PMID: 35513717
- PMCID: PMC9592120
- DOI: 10.1038/s41580-022-00479-6
Plant hormone regulation of abiotic stress responses
Erratum in
-
Publisher Correction: Plant hormone regulation of abiotic stress responses.Nat Rev Mol Cell Biol. 2022 Jul;23(7):516. doi: 10.1038/s41580-022-00501-x. Nat Rev Mol Cell Biol. 2022. PMID: 35637415 No abstract available.
Abstract
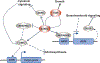
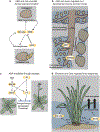
Plant hormones are signalling compounds that regulate crucial aspects of growth, development and environmental stress responses. Abiotic stresses, such as drought, salinity, heat, cold and flooding, have profound effects on plant growth and survival. Adaptation and tolerance to such stresses require sophisticated sensing, signalling and stress response mechanisms. In this Review, we discuss recent advances in understanding how diverse plant hormones control abiotic stress responses in plants and highlight points of hormonal crosstalk during abiotic stress signalling. Control mechanisms and stress responses mediated by plant hormones including abscisic acid, auxin, brassinosteroids, cytokinins, ethylene and gibberellins are discussed. We discuss new insights into osmotic stress sensing and signalling mechanisms, hormonal control of gene regulation and plant development during stress, hormone-regulated submergence tolerance and stomatal movements. We further explore how innovative imaging approaches are providing insights into single-cell and tissue hormone dynamics. Understanding stress tolerance mechanisms opens new opportunities for agricultural applications.
© 2022. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
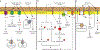

References
-
- Hamann E et al. Review: Plant eco-evolutionary responses to climate change: Emerging directions. Plant Sci 304, 110737 (2021). - PubMed
-
- Waadt R Phytohormone signaling mechanisms and genetic methods for their modulation and detection. Curr. Opin. Plant Biol 57, 31–40 (2020). - PubMed
-
- Zhao Y et al. Control of plant water use by ABA induction of senescence and dormancy: An overlooked lesson from evolution. Plant Cell Physiol 58, 1319–1327 (2017). - PubMed
-
- Yoshida T, Mogami J & Yamaguchi-Shinozaki K ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol 21, 133–139 (2014). - PubMed

