Immunogenicity and reactogenicity after booster dose with AZD1222 via intradermal route among adult who had received CoronaVac
- PMID: 35513961
- PMCID: PMC9058819
- DOI: 10.1016/j.vaccine.2022.04.067
Immunogenicity and reactogenicity after booster dose with AZD1222 via intradermal route among adult who had received CoronaVac
Abstract
Background: Currently, booster dose is needed after 2 doses of non-live COVID-19 vaccine. With limited resources and shortage of COVID-19 vaccines, intradermal(ID) administration might be a potential dose-sparing strategy.
Objective: To determine immunologic response and reactogenicity of ID ChAdOx1 nCoV-19 vaccine (AZD1222,Oxford/AstraZeneca) as a booster dose after completion of 2-dose CoronaVac(SV) in healthy adult.
Methods: This is a prospective cohort study of adult aged 18-59 years who received 2-dose SV at 14-35 days apart for more than 2 months. Participants received ID AZD1222 at fractional low dose(1×1010 viral particles,0.1 ml). Antibody responses were evaluated by surrogate virus neutralization test(sVNT) against delta variant and wild type, and anti-spike-receptor-binding-domain immunoglobulin G(anti-S-RBD IgG) at prior, day14, 28, 90, and 180 post booster. Solicited reactogenicity was collected for 7 days post-booster. Primary endpoint was the differences of sVNT against delta strain ≥ 80% inhibition at day14 and 90 compared with the parallel cohort study of 0.5-ml intramuscular(IM) route.
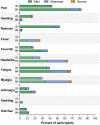
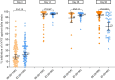
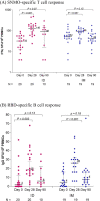
Results: From August2021, 100 adults with median age of 46 years(IQR 41-52) participated. Prior to booster, geometric mean(GM) of sVNT against delta strain was 22.4% inhibition(95 %CI 18.7-26.9) and of anti-S-RBD IgG was 109.3 BAU/ml(95.4-125.1). Post ID booster, GMs of sVNT against delta strain were 95.5% inhibition (95%CI 94.2-96.8) at day14, 73.1% inhibition (66.7-80.2) at day90, and 22.7% inhibition (14.9-34.6) at day180. The differences of proportion of participants achieving sVNT against delta strain ≥ 80% inhibition in ID recipients versus IM were + 4.2% (95 %CI -2.0to10.5) at day14, and -37.3%(-54.2to-20.3) at day90. Anti-S-RBD IgG GMs were 2037.1 BAU/ml (95%CI 1770.9-2343.2) at day14 and 744.6 BAU/ml(650.1-852.9) at day90, respectively. Geometric mean ratios(GMRs) of anti-S-RBD IgG were 0.99(0.83-1.20) at day14, and 0.82(0.66-1.02) at day90. Only 18% reported feverish, compared with 37% of IM (p = 0.003). Common reactogenicity was erythema at injection site(53%) while 7% reported blister.
Conclusion: Low-dose ID AZD1222 booster enhanced lower neutralizing antibodies at 3 months compared with IM route. Less systemic reactogenicity occurred, but higher local reactogenicity.
Keywords: AZD1222; Anti-SARS-CoV-2 IgG; Booster dose; ChAdOx1 nCoV-19 vaccine; CoronaVac vaccine; Intradermal; Neutralizing antibody titer; SARS-CoV-2 vaccine.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
A randomized clinical trial of a booster dose with low versus standard dose of AZD1222 in adult after 2 doses of inactivated vaccines.Vaccine. 2022 Apr 20;40(18):2551-2560. doi: 10.1016/j.vaccine.2022.03.036. Epub 2022 Mar 24. Vaccine. 2022. PMID: 35341647 Free PMC article. Clinical Trial.
-
Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study.Lancet. 2022 Feb 5;399(10324):521-529. doi: 10.1016/S0140-6736(22)00094-0. Epub 2022 Jan 21. Lancet. 2022. PMID: 35074136 Free PMC article. Clinical Trial.
-
The immunogenicity and reactogenicity of four COVID-19 booster vaccinations against SARS-CoV-2 variants following CoronaVac or ChAdOx1 nCoV-19 primary series.Asian Pac J Allergy Immunol. 2024 Sep;42(3):276-289. doi: 10.12932/AP-160123-1533. Asian Pac J Allergy Immunol. 2024. PMID: 37466962
-
Reactogenicity and immunogenicity of heterologous prime-boost immunization with COVID-19 vaccine.Biomed Pharmacother. 2022 Mar;147:112650. doi: 10.1016/j.biopha.2022.112650. Epub 2022 Jan 19. Biomed Pharmacother. 2022. PMID: 35066301 Free PMC article. Review.
-
Kinetics of anti-SARS-CoV-2 responses post complete vaccination with coronavac: A prospective study in 50 health workers.J Public Health Res. 2022 Aug 9;11(3):22799036221104173. doi: 10.1177/22799036221104173. eCollection 2022 Jul. J Public Health Res. 2022. PMID: 35966047 Free PMC article. Review.
Cited by
-
Immunogenicity of Intradermal Versus Intramuscular BNT162b2 COVID-19 Booster Vaccine in Patients with Immune-Mediated Dermatologic Diseases: A Non-Inferiority Randomized Controlled Trial.Vaccines (Basel). 2024 Jan 11;12(1):73. doi: 10.3390/vaccines12010073. Vaccines (Basel). 2024. PMID: 38250886 Free PMC article.
-
Reactogenicity and immunogenicity of the intradermal administration of BNT162b2 mRNA vaccine in healthy adults who were primed with an inactivated SARS-CoV-2 vaccine.Vaccine X. 2022 Nov 17;12:100242. doi: 10.1016/j.jvacx.2022.100242. eCollection 2022 Dec. Vaccine X. 2022. PMID: 36415450 Free PMC article.
-
SARS-CoV-2 Antibody Dynamics after COVID-19 Vaccination and Infection: A Real-World Cross-Sectional Analysis.Vaccines (Basel). 2023 Jun 30;11(7):1184. doi: 10.3390/vaccines11071184. Vaccines (Basel). 2023. PMID: 37515001 Free PMC article.
-
Immunogenicity of BNT162b2 in children 6 months to under 5 years of age with previous SARS-CoV-2 infection, in the era of Omicron predominance.Vaccine X. 2023 Aug 5;15:100367. doi: 10.1016/j.jvacx.2023.100367. eCollection 2023 Dec. Vaccine X. 2023. PMID: 37601322 Free PMC article.
-
Heterologous Prime-boost of SARS-CoV-2 inactivated vaccine and mRNA BNT162b2 among Healthy Thai Adolescents.Vaccine X. 2022 Dec;12:100211. doi: 10.1016/j.jvacx.2022.100211. Epub 2022 Aug 29. Vaccine X. 2022. PMID: 36059600 Free PMC article.
References
-
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard [Available from: https://covid19.who.int/. Accessed date 28 November, 2021.
-
- Hitchings M.D.T., Ranzani O.T., Torres M.S.S., de Oliveira S.B., Almiron M., Said R., et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 Gamma variant transmission in Manaus, Brazil: A test-negative case-control study. Lancet Reg Health Am. 2021;1:100025. - PMC - PubMed
-
- Liu X., Shaw R.H., Stuart A.S.V., Greenland M., Aley P.K., Andrews N.J., et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398(10303):856–869. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

