Antibody Duration After Infection From SARS-CoV-2 in the Texas Coronavirus Antibody Response Survey
- PMID: 35514141
- PMCID: PMC9833436
- DOI: 10.1093/infdis/jiac167
Antibody Duration After Infection From SARS-CoV-2 in the Texas Coronavirus Antibody Response Survey
Abstract
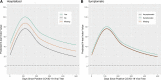
Understanding the duration of antibodies to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus that causes COVID-19 is important to controlling the current pandemic. Participants from the Texas Coronavirus Antibody Response Survey (Texas CARES) with at least 1 nucleocapsid protein antibody test were selected for a longitudinal analysis of antibody duration. A linear mixed model was fit to data from participants (n = 4553) with 1 to 3 antibody tests over 11 months (1 October 2020 to 16 September 2021), and models fit showed that expected antibody response after COVID-19 infection robustly increases for 100 days postinfection, and predicts individuals may remain antibody positive from natural infection beyond 500 days depending on age, body mass index, smoking or vaping use, and disease severity (hospitalized or not; symptomatic or not).
Keywords: COVID-19; SARS-CoV-2; antibodies; antibody duration.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

