The balance of interleukin-12 and interleukin-23 determines the bias of MAIT1 versus MAIT17 responses during bacterial infection
- PMID: 35514192
- PMCID: PMC9539875
- DOI: 10.1111/imcb.12556
The balance of interleukin-12 and interleukin-23 determines the bias of MAIT1 versus MAIT17 responses during bacterial infection
Abstract
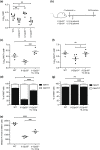
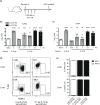
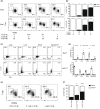
Mucosal-associated invariant T (MAIT) cells are a major subset of innate-like T cells mediating protection against bacterial infection through recognition of microbial metabolites derived from riboflavin biosynthesis. Mouse MAIT cells egress from the thymus as two main subpopulations with distinct functions, namely, T-bet-expressing MAIT1 and RORγt-expressing MAIT17 cells. Previously, we reported that inducible T-cell costimulator and interleukin (IL)-23 provide essential signals for optimal MHC-related protein 1 (MR1)-dependent activation and expansion of MAIT17 cells in vivo. Here, in a model of tularemia, in which MAIT1 responses predominate, we demonstrate that IL-12 and IL-23 promote MAIT1 cell expansion during acute infection and that IL-12 is indispensable for MAIT1 phenotype and function. Furthermore, we showed that the bias toward MAIT1 or MAIT17 responses we observed during different bacterial infections was determined and modulated by the balance between IL-12 and IL-23 and that these responses could be recapitulated by cytokine coadministration with antigen. Our results indicate a potential for tailored immunotherapeutic interventions via MAIT cell manipulation.
Keywords: IL-12; IL-23; MAIT cells; bacterial infection; mucosal-associated invariant T cells.
© 2022 The Authors. Immunology & Cell Biology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
SBGE, JYWM, DPF, JM, AJC and ZC are inventors on patents (WO2014/005194 and WO2015/149130) describing MR1 tetramers and MR1 ligands. The other authors declare that they have no competing interests.
Figures


Similar articles
-
MicroRNA-155 Regulates MAIT1 and MAIT17 Cell Differentiation.Front Cell Dev Biol. 2021 Apr 9;9:670531. doi: 10.3389/fcell.2021.670531. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33898469 Free PMC article.
-
MAIT cell plasticity enables functional adaptation that drives antibacterial immune protection.Sci Immunol. 2024 Dec 6;9(102):eadp9841. doi: 10.1126/sciimmunol.adp9841. Epub 2024 Dec 6. Sci Immunol. 2024. PMID: 39642244
-
Differential controls of MAIT cell effector polarization by mTORC1/mTORC2 via integrating cytokine and costimulatory signals.Nat Commun. 2021 Apr 1;12(1):2029. doi: 10.1038/s41467-021-22162-8. Nat Commun. 2021. PMID: 33795689 Free PMC article.
-
MHC class I-related molecule, MR1, and mucosal-associated invariant T cells.Immunol Rev. 2016 Jul;272(1):120-38. doi: 10.1111/imr.12423. Immunol Rev. 2016. PMID: 27319347 Review.
-
MAIT Cell Activation and Functions.Front Immunol. 2020 May 27;11:1014. doi: 10.3389/fimmu.2020.01014. eCollection 2020. Front Immunol. 2020. PMID: 32536923 Free PMC article. Review.
Cited by
-
Protective effect of TCR-mediated MAIT cell activation during experimental autoimmune encephalomyelitis.Nat Commun. 2024 Oct 28;15(1):9287. doi: 10.1038/s41467-024-53657-9. Nat Commun. 2024. PMID: 39468055 Free PMC article.
-
Single-cell map of innate-like lymphocyte response to Francisella tularensis infection reveals interleukin-17-dependent protection by MAIT cells.iScience. 2025 Jan 16;28(3):111810. doi: 10.1016/j.isci.2025.111810. eCollection 2025 Mar 21. iScience. 2025. PMID: 40160424 Free PMC article.
-
Editorial: MAIT cells come of age.Front Immunol. 2023 Sep 6;14:1281881. doi: 10.3389/fimmu.2023.1281881. eCollection 2023. Front Immunol. 2023. PMID: 37744347 Free PMC article. No abstract available.
-
Synthetic 5-amino-6-D-ribitylaminouracil paired with inflammatory stimuli facilitates MAIT cell expansion in vivo.Front Immunol. 2023 Aug 31;14:1109759. doi: 10.3389/fimmu.2023.1109759. eCollection 2023. Front Immunol. 2023. PMID: 37720229 Free PMC article.
-
Classic costimulatory interactions in MAIT cell responses: from gene expression to immune regulation.Clin Exp Immunol. 2023 Jul 5;213(1):50-66. doi: 10.1093/cei/uxad061. Clin Exp Immunol. 2023. PMID: 37279566 Free PMC article.
References
-
- McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity 2008; 28: 445–453. - PubMed
-
- Ruterbusch M, Pruner KB, Shehata L, Pepper M. In vivo CD4+ T cell differentiation and function: revisiting the Th1/Th2 paradigm. Annu Rev Immunol 2020; 38: 705–725. - PubMed
-
- Lepore M, Kalinichenko A, Colone A, et al. Parallel T‐cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat Commun 2014; 5: 3866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

