The role of Lrp6-mediated Wnt/β-catenin signaling in the development and intervention of spinal neural tube defects in mice
- PMID: 35514236
- PMCID: PMC9194482
- DOI: 10.1242/dmm.049517
The role of Lrp6-mediated Wnt/β-catenin signaling in the development and intervention of spinal neural tube defects in mice
Abstract
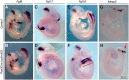
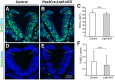
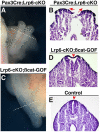
Neural tube defects (NTDs) are among the common and severe birth defects with poorly understood etiology. Mutations in the Wnt co-receptor LRP6 are associated with NTDs in humans. Either gain-of-function (GOF) or loss-of-function (LOF) mutations of Lrp6 can cause NTDs in mice. NTDs in Lrp6-GOF mutants may be attributed to altered β-catenin-independent noncanonical Wnt signaling. However, the mechanisms underlying NTDs in Lrp6-LOF mutants and the role of Lrp6-mediated canonical Wnt/β-catenin signaling in neural tube closure remain unresolved. We previously demonstrated that β-catenin signaling is required for posterior neuropore (PNP) closure. In the current study, conditional ablation of Lrp6 in dorsal PNP caused spinal NTDs with diminished activities of Wnt/β-catenin signaling and its downstream target gene Pax3, which is required for PNP closure. β-catenin-GOF rescued NTDs in Lrp6-LOF mutants. Moreover, maternal supplementation of a Wnt/β-catenin signaling agonist reduced the frequency and severity of spinal NTDs in Lrp6-LOF mutants by restoring Pax3 expression. Together, these results demonstrate the essential role of Lrp6-mediated Wnt/β-catenin signaling in PNP closure, which could also provide a therapeutic target for NTD intervention through manipulation of canonical Wnt/β-catenin signaling activities.
Keywords: Genetic rescue; Lrp6; Pharmacological intervention; Spinal neural tube defects; Wnt/β-catenin signaling.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures





Similar articles
-
β-catenin regulates Pax3 and Cdx2 for caudal neural tube closure and elongation.Development. 2014 Jan;141(1):148-57. doi: 10.1242/dev.101550. Epub 2013 Nov 27. Development. 2014. PMID: 24284205 Free PMC article.
-
Novel Mutation of LRP6 Identified in Chinese Han Population Links Canonical WNT Signaling to Neural Tube Defects.Birth Defects Res. 2018 Jan 15;110(1):63-71. doi: 10.1002/bdr2.1122. Epub 2017 Sep 29. Birth Defects Res. 2018. PMID: 28960852
-
Genetic interaction of Pax3 mutation and canonical Wnt signaling modulates neural tube defects and neural crest abnormalities.Genesis. 2021 Nov;59(11):e23445. doi: 10.1002/dvg.23445. Epub 2021 Sep 7. Genesis. 2021. PMID: 34490995
-
β-Catenin-Independent Roles of Wnt/LRP6 Signaling.Trends Cell Biol. 2016 Dec;26(12):956-967. doi: 10.1016/j.tcb.2016.07.009. Epub 2016 Aug 24. Trends Cell Biol. 2016. PMID: 27568239 Review.
-
Planar cell polarity gene mutations contribute to the etiology of human neural tube defects in our population.Birth Defects Res A Clin Mol Teratol. 2014 Aug;100(8):633-41. doi: 10.1002/bdra.23255. Epub 2014 May 17. Birth Defects Res A Clin Mol Teratol. 2014. PMID: 24838524 Review.
Cited by
-
Parental arsenic exposure and tissue-specific DNA methylation in Bangladeshi infants with spina bifida.Epigenetics. 2024 Dec;19(1):2416345. doi: 10.1080/15592294.2024.2416345. Epub 2024 Oct 19. Epigenetics. 2024. PMID: 39425535 Free PMC article.
-
The significance of calcium ions in cerebral ischemia-reperfusion injury: mechanisms and intervention strategies.Front Mol Biosci. 2025 May 12;12:1585758. doi: 10.3389/fmolb.2025.1585758. eCollection 2025. Front Mol Biosci. 2025. PMID: 40421420 Free PMC article. Review.
-
A rationally designed optochemogenetic switch for activating canonical Wnt signaling.iScience. 2023 Feb 19;26(3):106233. doi: 10.1016/j.isci.2023.106233. eCollection 2023 Mar 17. iScience. 2023. PMID: 36915690 Free PMC article.
-
Balancing WNT signalling in early forebrain development: The role of LRP4 as a modulator of LRP6 function.Front Cell Dev Biol. 2023 Apr 7;11:1173688. doi: 10.3389/fcell.2023.1173688. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37091972 Free PMC article.
-
Epigenetic regulation by TET1 in gene-environmental interactions influencing susceptibility to congenital malformations.bioRxiv [Preprint]. 2024 Jul 8:2024.02.21.581196. doi: 10.1101/2024.02.21.581196. bioRxiv. 2024. PMID: 39026762 Free PMC article. Preprint.
References
-
- Allache, R., Lachance, S., Guyot, M. C., De Marco, P., Merello, E., Justice, M. J., Capra, V. and Kibar, Z. (2014). Novel mutations in Lrp6 orthologs in mouse and human neural tube defects affect a highly dosage-sensitive Wnt non-canonical planar cell polarity pathway. Hum. Mol. Genet. 23, 1687-1699. 10.1093/hmg/ddt558 - DOI - PMC - PubMed
-
- Bryja, V., Andersson, E. R., Schambony, A., Esner, M., Bryjova, L., Biris, K. K., Hall, A. C., Kraft, B., Cajanek, L., Yamaguchi, T. P.et al. (2009). The extracellular domain of Lrp5/6 inhibits noncanonical Wnt signaling in vivo. Mol. Biol. Cell 20, 924-936. 10.1091/mbc.e08-07-0711 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

