Autophagy is Involved in Cardiac Remodeling in Response to Environmental Temperature Change
- PMID: 35514342
- PMCID: PMC9061941
- DOI: 10.3389/fphys.2022.864427
Autophagy is Involved in Cardiac Remodeling in Response to Environmental Temperature Change
Abstract
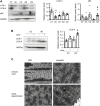
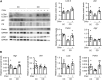
Objectives: To study the reversibility of cold-induced cardiac hypertrophy and the role of autophagy in this process. Background: Chronic exposure to cold is known to cause cardiac hypertrophy independent of blood pressure elevation. The reversibility of this process and the molecular mechanisms involved are unknown. Methods: Studies were performed in two-month-old mice exposed to cold (4°C) for 24 h or 10 days. After exposure, the animals were returned to room temperature (21°C) for 24 h or 1 week. Results: We found that chronic cold exposure significantly increased the heart weight/tibia length (HW/TL) ratio, the mean area of cardiomyocytes, and the expression of hypertrophy markers, but significantly decreased the expression of genes involved in fatty acid oxidation. Echocardiographic measurements confirmed hypertrophy development after chronic cold exposure. One week of deacclimation for cold-exposed mice fully reverted the morphological, functional, and gene expression indicators of cardiac hypertrophy. Experiments involving injection of leupeptin at 1 h before sacrifice (to block autophagic flux) indicated that cardiac autophagy was repressed under cold exposure and re-activated during the first 24 h after mice were returned to room temperature. Pharmacological blockage of autophagy for 1 week using chloroquine in mice subjected to deacclimation from cold significantly inhibited the reversion of cardiac hypertrophy. Conclusion: Our data indicate that mice exposed to cold develop a marked cardiac hypertrophy that is reversed after 1 week of deacclimation. We propose that autophagy is a major mechanism underlying the heart remodeling seen in response to cold exposure and its posterior reversion after deacclimation.
Keywords: autophagy; heart; hypertrophy; metabolism; temperature.
Copyright © 2022 Ruperez, Blasco-Roset, Kular, Cairo, Ferrer-Curriu, Villarroya, Zamora, Crispi, Villarroya and Planavila.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures