A Neural Crest-specific Overexpression Mouse Model Reveals the Transcriptional Regulatory Effects of Dlx2 During Maxillary Process Development
- PMID: 35514355
- PMCID: PMC9070692
- DOI: 10.3389/fphys.2022.855959
A Neural Crest-specific Overexpression Mouse Model Reveals the Transcriptional Regulatory Effects of Dlx2 During Maxillary Process Development
Abstract
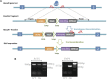
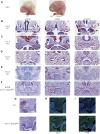
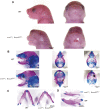
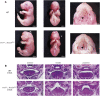
Craniofacial morphogenesis is a complex process that requires precise regulation of cell proliferation, migration, and differentiation. Perturbations of this process cause a series of craniofacial deformities. Dlx2 is a critical transcription factor that regulates the development of the first branchial arch. However, the transcriptional regulatory functions of Dlx2 during craniofacial development have been poorly understood due to the lack of animal models in which the Dlx2 level can be precisely modulated. In this study, we constructed a Rosa26 site-directed Dlx2 gene knock-in mouse model Rosa26 CAG-LSL-Dlx2-3xFlag for conditionally overexpressing Dlx2. By breeding with wnt1 cre mice, we obtained wnt1 cre ; Rosa26 Dlx2/- mice, in which Dlx2 is overexpressed in neural crest lineage at approximately three times the endogenous level. The wnt1 cre ; Rosa26 Dlx2/- mice exhibited consistent phenotypes that include cleft palate across generations and individual animals. Using this model, we demonstrated that Dlx2 caused cleft palate by affecting maxillary growth and uplift in the early-stage development of maxillary prominences. By performing bulk RNA-sequencing, we demonstrated that Dlx2 overexpression induced significant changes in many genes associated with critical developmental pathways. In summary, our novel mouse model provides a reliable and consistent system for investigating Dlx2 functions during development and for elucidating the gene regulatory networks underlying craniofacial development.
Keywords: Dlx2; RNA-seq; cleft palate; cranial neural crest cells; craniofacial development.
Copyright © 2022 Sun, Ha, Liu, Bian and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Carre A. L., James A. W., MacLeod L., Kong W., Kawai K., Longaker M. T., et al. (2010). Interaction of Wingless Protein (Wnt), Transforming Growth Factor-Β1, and Hyaluronan Production in Fetal and Postnatal Fibroblasts. Plast. Reconstr. Surg. 125 (1), 74–88. 10.1097/PRS.0b013e3181c495d1 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

