Molecular docking analysis and anti-hyperglycaemic activity of Synacinn™ in streptozotocin-induced rats
- PMID: 35514405
- PMCID: PMC9058594
- DOI: 10.1039/d0ra04664g
Molecular docking analysis and anti-hyperglycaemic activity of Synacinn™ in streptozotocin-induced rats
Abstract
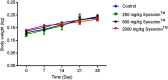
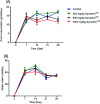
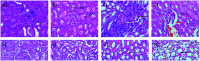
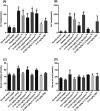
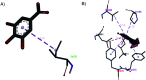
Synacinn™ is a standardized polyherbal supplement formulated from Cinnamomum zeylanicum Blume, Curcuma zanthorrhiza Roxb., Syzygium polyanthum (Wight) Walp., Orthosiphon stamineus Benth. and Andrographis paniculata (Burm.f.) Nees. It is designed for the synergistic treatment of diabetes mellitus and its complications. Although the beneficial effects are yet to be verified scientifically, it is traditionally used to improve general health in patients with diabetes. This study aimed to evaluate the anti-hyperglycemic effects of Synacinn™ in a streptozotocin-induced type 1 diabetes rat model. Initially, Synacinn™ was used for in vivo acute oral toxicity tests and 14 day repeated dose toxicity tests to determine the toxicity levels. An efficacy study of Synacinn™ was carried out via the oral administration of 10, 50, 100, 250, and 250 (b.i.d.) mg kg-1 doses to streptozotocin-induced diabetic rats. After 28 days, blood serum was collected to measure the fasting blood glucose, triglyceride, cholesterol, alanine aminotransferase, alkaline phosphatase, creatinine, and uric acid levels. The liver, kidney, and pancreas structures were histopathologically analyzed. In silico binding interaction studies of five phytochemicals in Synacinn™ identified via HPLC with glucokinase were performed using molecular docking analysis. The results showed that although no mortality was observed during the acute oral toxicity tests, notable damage to the liver and kidney occurred during the 14 day repeated dose testing at Synacinn™ levels of 600 mg kg-1 and 2000 mg kg-1. Treatment with 250 mg kg-1 (b.i.d.) Synacinn™ of the streptozotocin-induced type 1 diabetic rats significantly (p < 0.05) improved the fasting blood glucose (59%), triglyceride (58%), cholesterol (47%), alanine aminotransferase (60%), alkaline phosphatase (90%), and creatinine (32%) levels. Synacinn™ also improved the relative weights of liver (35%), kidney (36%), and pancreatic (36%) tissue. Histological analysis showed improvements in the conditions of the central vein of the liver, the kidney Bowman's capsule and glomerulus, and the pancreatic islets of Langerhans. HPLC analysis of a standardized extract identified five active phytochemicals: andrographolide (17.36 mg g-1), gallic acid (11.5 mg g-1), curcumin (2.75 mg g-1), catechin (3.9 mg g-1), and rosmarinic acid (5.54 mg g-1). Molecular docking studies with glucokinase showed that andrographolide yields the highest binding energy (-12.1 kcal mol-1), followed by catechin (-10.2 kcal mol-1), rosmarinic acid (-8.6 kcal mol-1), curcumin (-7.8 kcal mol-1), and gallic acid (-5.6 kcal mol-1). These current findings suggest that Synacinn™ at a dose of 250 mg kg-1 was non-toxic to rats. A twice-daily 250 mg kg-1 dose of Synacinn™ is an effective anti-hyperglycemic agent, lowering blood glucose, triglyceride, and cholesterol levels, and assisting the recovery of organ impairment caused by streptozotocin in type 1 diabetic rats.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures










Similar articles
-
Phytochemical screening and preliminary clinical trials of the aqueous extract mixture of Andrographis paniculata (Burm. f.) Wall. ex Nees and Syzygium polyanthum (Wight.) Walp leaves in metformin treated patients with type 2 diabetes.Phytomedicine. 2019 Mar 1;55:137-147. doi: 10.1016/j.phymed.2018.07.002. Epub 2018 Jul 21. Phytomedicine. 2019. PMID: 30668423 Clinical Trial.
-
Triple-action of the standardized antidiabetic polyherbal extract; Synacinn™ through upregulation of GLUT4 and inhibition of DPP(IV), α-amylase, and α-glucosidase activity.Med J Malaysia. 2022 Jul;77(Suppl 1):16-22. Med J Malaysia. 2022. PMID: 35899882
-
Nephroprotective effect of Combretum micranthum G. Don in nicotinamide-streptozotocin induced diabetic nephropathy in rats: In-vivo and in-silico experiments.J Ethnopharmacol. 2020 Oct 28;261:113133. doi: 10.1016/j.jep.2020.113133. Epub 2020 Jul 14. J Ethnopharmacol. 2020. PMID: 32673708
-
Pancreatic effect of andrographolide isolated from Andrographis paniculata (Burm. f.) Nees.Pak J Biol Sci. 2014 Jan 1;17(1):22-31. doi: 10.3923/pjbs.2014.22.31. Pak J Biol Sci. 2014. PMID: 24783774
-
Antidiabetic and antihiperlipidemic effect of Andrographis paniculata (Burm. f.) Nees and andrographolide in high-fructose-fat-fed rats.Indian J Pharmacol. 2012 May;44(3):377-81. doi: 10.4103/0253-7613.96343. Indian J Pharmacol. 2012. PMID: 22701250 Free PMC article.
Cited by
-
Mutagenicity and safety pharmacology of a standardized antidiabetic polyherbal formulation.Sci Rep. 2022 May 3;12(1):7127. doi: 10.1038/s41598-022-11243-3. Sci Rep. 2022. PMID: 35505003 Free PMC article.
-
Dual actions of gallic acid and andrographolide trigger AdipoR1 to stimulate insulin secretion in a streptozotocin-induced diabetes rat model.J Tradit Complement Med. 2022 Sep 28;13(1):11-19. doi: 10.1016/j.jtcme.2022.09.002. eCollection 2023 Jan. J Tradit Complement Med. 2022. PMID: 36685073 Free PMC article.
-
Gastroprotective effect of rhodanine and 2,4-thiazolidinediones scaffolds in rat stomachs by contribution of anti-apoptotic (BCL-2) and tumor suppressor (P53) proteins.Sci Rep. 2024 Jan 19;14(1):1699. doi: 10.1038/s41598-024-51446-4. Sci Rep. 2024. PMID: 38242960 Free PMC article.
-
Onopordum acanthium L. extract attenuates pancreatic β-Cells and cardiac inflammation in streptozocin-induced diabetic rats.PLoS One. 2023 Jan 25;18(1):e0280464. doi: 10.1371/journal.pone.0280464. eCollection 2023. PLoS One. 2023. PMID: 36696433 Free PMC article.
-
In silico and in vivo hepatoprotective activity of the synthesized 5-benzylidene-2-thiohydantoin against diethylnitrosamine-induced liver injury in a rat model.Sci Rep. 2023 Mar 22;13(1):4681. doi: 10.1038/s41598-023-27725-x. Sci Rep. 2023. PMID: 36949140 Free PMC article.
References
-
- Sharma A. Gupta R. J. Diabetes Metab. 2017;8(7):1–7.
-
- Mukhtar H. Wadhan P. Singh V. J. Nat. Rem. 2013;13:9–14.
-
- Park C. Lee J. S. Biomed. Res. 2013;24:164–169.
LinkOut - more resources
Full Text Sources

