Carotane sesquiterpenes from Ferula vesceritensis: in silico analysis as SARS-CoV-2 binding inhibitors
- PMID: 35514418
- PMCID: PMC9056801
- DOI: 10.1039/d0ra06901a
Carotane sesquiterpenes from Ferula vesceritensis: in silico analysis as SARS-CoV-2 binding inhibitors
Abstract
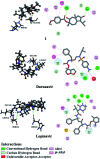
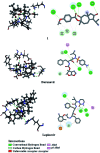
Two sesquiterpenes, 8α-anisate-dauc-4-ene-3,9-dione (webiol anisate) (1) and 10α-acetoxy-6α-benzoate-jaeschkeanadiol (2) as well as, ten known analogues (3-10), and two sesquiterpene coumarins (11-12) were isolated from an organic root extract of Ferula vesceritensis (Fam. Apiaceae). Chemical structures were elucidated based on IR, 1D- and 2D-NMR and HRMS, spectroscopic analyses. With molecular overlap observed between two protease inhibitors that are being examined as anti-COVID-19 drugs, and sesquiterpenes isolated here, metabolite molecular docking calculations were made using the main protease (Mpro), which is required for viral multiplication as well as RNA-dependent RNA polymerase (RdRp). In silico binding-inhibition analysis predicted that select F. vesceritensis sesquiterpenes can bind to these enzymes required for viral replication. Structures of the isolated constituents were also consistent with the chemo-systematic grouping of F. vesceritensis secondary metabolites with other Ferula species.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Sesquiterpenes from aerial parts of Ferula vesceritensis.Phytochemistry. 2008 Jun;69(9):1933-8. doi: 10.1016/j.phytochem.2008.03.010. Epub 2008 May 14. Phytochemistry. 2008. PMID: 18485426
-
A crystal lapiferin derived from Ferula vesceritensis induces apoptosis pathway in MCF-7 breast cancer cells.Nat Prod Res. 2010 Feb;24(3):246-57. doi: 10.1080/14786410802685398. Nat Prod Res. 2010. PMID: 20140803
-
Feruhermonins A-C: three daucane esters from the seeds of Ferula hermonis (Apiaceae).J Asian Nat Prod Res. 2008 Jul-Aug;10(7-8):711-7. doi: 10.1080/10286020802016040. J Asian Nat Prod Res. 2008. PMID: 18696321
-
Biologically active sesquiterpene coumarins from Ferula species.Phytother Res. 2011 Mar;25(3):315-23. doi: 10.1002/ptr.3311. Epub 2010 Oct 28. Phytother Res. 2011. PMID: 21031633 Review.
-
Sesquiterpenes and Sesquiterpene Derivatives from Ferula: Their Chemical Structures, Biosynthetic Pathways, and Biological Properties.Antioxidants (Basel). 2023 Dec 19;13(1):7. doi: 10.3390/antiox13010007. Antioxidants (Basel). 2023. PMID: 38275627 Free PMC article. Review.
Cited by
-
Comparative Study on the Essential Oils from Five Wild Egyptian Centaurea Species: Effective Extraction Techniques, Antimicrobial Activity and In-Silico Analyses.Antibiotics (Basel). 2021 Mar 3;10(3):252. doi: 10.3390/antibiotics10030252. Antibiotics (Basel). 2021. PMID: 33802470 Free PMC article.
-
Gastroprotection against Rat Ulcers by Nephthea Sterol Derivative.Biomolecules. 2021 Aug 21;11(8):1247. doi: 10.3390/biom11081247. Biomolecules. 2021. PMID: 34439913 Free PMC article.
-
Two novel oxetane containing lignans and a new megastigmane from Paronychia arabica and in silico analysis of them as prospective SARS-CoV-2 inhibitors.RSC Adv. 2021 Jun 4;11(33):20151-20163. doi: 10.1039/d1ra02486h. eCollection 2021 Jun 3. RSC Adv. 2021. PMID: 35479905 Free PMC article.
-
Antiviral Effect of Ferula Plants and their Potential for Treatment of COVID-19: A Comprehensive Review.Curr Pharm Biotechnol. 2025;26(8):1221-1231. doi: 10.2174/0113892010285343240530040218. Curr Pharm Biotechnol. 2025. PMID: 38967074 Review.
-
Discovering new potential inhibitors to SARS-CoV-2 RNA dependent RNA polymerase (RdRp) using high throughput virtual screening and molecular dynamics simulations.Sci Rep. 2022 Nov 21;12(1):19986. doi: 10.1038/s41598-022-24695-4. Sci Rep. 2022. PMID: 36411383 Free PMC article.
References
-
- Boulos L., Medicinal Plants of North Africa, Reference Publications, Algonac, 1983, p. 183
-
- Yaqoob U. Nawchoo I. A. J. King Saud Univ., Sci. 2017;29:19–27. doi: 10.1016/j.jksus.2015.10.002. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

