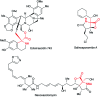
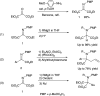
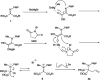
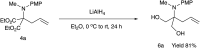Double nucleophilic addition to iminomalonate, leading to the synthesis of quaternary α-amino diesters and desymmetrization of the products
- PMID: 35514493
- PMCID: PMC9067299
- DOI: 10.1039/c9ra04889h
Double nucleophilic addition to iminomalonate, leading to the synthesis of quaternary α-amino diesters and desymmetrization of the products
Abstract
Alkylation of iminomalonate with Grignard reagents followed by oxidation and allylation gave symmetrical quaternary α-amino diesters in good yields. Subsequent desymmetrization of a diol derivative from these products was conducted via asymmetric carbamylation catalyzed by Cu-Bnbox to give chiral quaternary aminodiol mono-carbamates.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Enantioselective synthesis of alpha-quaternary amino acid derivatives by sequential enzymatic desymmetrization and Curtius rearrangement of alpha,alpha-disubstituted malonate diesters.J Org Chem. 2010 Mar 5;75(5):1612-9. doi: 10.1021/jo902584r. J Org Chem. 2010. PMID: 20131857
-
Stereodivergent Desymmetrization of Simple Dicarboxylates via Branch-Selective Pd/Cu Catalyzed Allylic Substitution.Chemistry. 2022 Apr 27;28(24):e202200273. doi: 10.1002/chem.202200273. Epub 2022 Mar 24. Chemistry. 2022. PMID: 35262998
-
Desymmetrization of Inert meso-Diethers through Copper-Catalyzed Asymmetric Allylic Alkylation with Grignard Reagents.Org Lett. 2024 Jul 12;26(27):5844-5849. doi: 10.1021/acs.orglett.4c01972. Epub 2024 Jul 1. Org Lett. 2024. PMID: 38950387
-
Asymmetric Synthesis of a Quaternary Carbon Stereogenic Center by Organocatalysis Using a Primary Amino Acid and Its Salt.Chem Rec. 2023 Jul;23(7):e202200276. doi: 10.1002/tcr.202200276. Epub 2023 Feb 2. Chem Rec. 2023. PMID: 36732858 Review.
-
Catalytic asymmetric desymmetrization approaches to enantioenriched cyclopentanes.Org Biomol Chem. 2015 Jan 7;13(1):18-24. doi: 10.1039/c4ob01649a. Org Biomol Chem. 2015. PMID: 25335849 Review.
Cited by
-
Preparation and facile addition reactions of iminium salts derived from amino ketene silyl acetal and amino silyl enol ether.RSC Adv. 2020 Jul 24;10(46):27874-27883. doi: 10.1039/d0ra05768a. eCollection 2020 Jul 21. RSC Adv. 2020. PMID: 35516926 Free PMC article.
-
An umpolung reaction of α-iminothioesters possessing a cyclopropyl group.RSC Adv. 2020 Mar 10;10(17):9955-9963. doi: 10.1039/d0ra01152e. eCollection 2020 Mar 6. RSC Adv. 2020. PMID: 35498585 Free PMC article.
-
N-Alkylation/aldol reaction of α-aldimino thioesters: a facile three-component coupling reaction.RSC Adv. 2021 Apr 7;11(22):13097-13104. doi: 10.1039/d1ra02000e. eCollection 2021 Apr 7. RSC Adv. 2021. PMID: 35423863 Free PMC article.
References
-
-
For reviews of α,α-disubstituted amino acids, see:
- Cativiela C. Diaz-de-Villegas M. D. Tetrahedron: Asymmetry. 1998;9:3517–3599. doi: 10.1016/S0957-4166(98)00391-7. - DOI
- Cativiela C. Diaz-de-Villegas M. D. Tetrahedron: Asymmetry. 2000;11:645–732. doi: 10.1016/S0957-4166(99)00565-0. - DOI
- Spino C. Angew. Chem., Int. Ed. 2004;43:1764–1766. doi: 10.1002/anie.200301686. - DOI - PubMed
- Ohfune Y. Shinada T. Eur. J. Org. Chem. 2005:5127–5143. doi: 10.1002/ejoc.200500434. - DOI - PubMed
- Vogt H. Brase S. Org. Biomol. Chem. 2007;5:406–430. doi: 10.1039/B611091F. - DOI - PubMed
- Calaza M. I. Cativiela C. Eur. J. Chem. 2008:3427–3448. - PMC - PubMed
- Cativiela C. Ordóñez M. Tetrahedron: Asymmetry. 2009;20:1–63. doi: 10.1016/j.tetasy.2009.01.002. - DOI - PMC - PubMed
-
-
-
For synthesis of α,α-disubstituted amino acids, see the followings. Alkylation of enolate:
- Belokon Y. N. Davies R. G. North M. Tetrahedron Lett. 2000;41:7245–7248. doi: 10.1016/S0040-4039(00)01247-8. - DOI
- Belokon Y. N. Bhave D. D'Addario D. Groaz E. North V. M. Pertrosyan A. Tetrahedron Lett. 2003;44:2045–2048. doi: 10.1016/S0040-4039(03)00170-9. - DOI
-
; Alkylation of cyclic compounds:
- Williams R. M. Im M.-N. J. Am. Chem. Soc. 1991;113:9276–9286. doi: 10.1021/ja00024a038. - DOI
- Berkowitz D. B. McFadden J. M. Chisowa E. Semerad C. L. J. Am. Chem. Soc. 2000;122:11031–11032. doi: 10.1021/ja0055110. - DOI - PMC - PubMed
-
; Asymmetric Strecker reaction:
- Masumoto S. Usuda H. Suzuki M. Kanai M. Shibasaki M. J. Am. Chem. Soc. 2003;125:5634–5635. doi: 10.1021/ja034980+. - DOI - PubMed
- Wang J. Hu X. Jiang J. Gou S. Huang X. Feng X. Angew. Chem., Int. Ed. 2007;46:8468–8470. doi: 10.1002/anie.200703188. - DOI - PubMed
-
; Addition to imino ester:
- Mitani M. Tanaka Y. Sawada A. Misu A. Matsumoto Y. Eur. J. Org. Chem. 2008:1383–1391. doi: 10.1002/ejoc.200700929. - DOI
-
; Radical reaction:
- Miyabe H. Asada R. Takemoto Y. Tetrahedron. 2005;61:385–393. doi: 10.1016/j.tet.2004.10.104. - DOI
-
; Memory of chirality:
- Kawabata T. Wirth T. Yahiro K. Suzuki H. Fuji K. J. Am. Chem. Soc. 1994;116:10809–10810. doi: 10.1021/ja00102a066. - DOI
- Fuji K. Kawabata T. Chem.–Eur. J. 1998;4:373–376. doi: 10.1002/(SICI)1521-3765(19980310)4:3<373::AID-CHEM373>3.0.CO;2-O. - DOI
- Kawabata T. Chen J. Suzuki H. Nagae Y. Kinoshita T. Chancharunee S. Fuji K. Org. Lett. 2000;2:3883–3885. doi: 10.1021/ol0066274. - DOI - PubMed
- Kawabata T. Kawakami S. Fuji K. Tetrahedron Lett. 2002;43:1465–1467. doi: 10.1016/S0040-4039(02)00034-5. - DOI
-
; Mitsunobu reaction:
- Green J. E. Bender D. M. Jackson S. O'Donnell M. J. McCarthy J. R. Org. Lett. 2009;11:807–810. doi: 10.1021/ol802325h. - DOI - PubMed
-
; Sigmatropic rearrangement:
- Anderson J. C. Skerrat S. J. Chem. Soc., Perkin Trans. 1. 2002:2871–2876. doi: 10.1039/B207295E. - DOI
-
; Beckmannn rearrangement:
- Frutos R. P. Spero D. M. Tetrahedron Lett. 1998;39:2475–2478. doi: 10.1016/S0040-4039(98)00309-8. - DOI
-
; Asymmetric hydrolysis:
- Iosub V. Haberl A. R. Leung J. Tang M. Vembaiyan K. Parvez M. Back T. G. J. Org. Chem. 2010;75:1612–1619. doi: 10.1021/jo902584r. - DOI - PubMed
-
-
- Corey E. J. Gin D. Y. Kania R. S. J. Am. Chem. Soc. 1996;118:9202–9203. doi: 10.1021/ja962480t. - DOI
-
- Kende A. S. Kawamura K. DeVita R. J. J. Am. Chem. Soc. 1990;112:4070–4072. doi: 10.1021/ja00166a072. - DOI
LinkOut - more resources
Full Text Sources