Linker length in fluorophore-cholesterol conjugates directs phase selectivity and cellular localisation in GUVs and live cells
- PMID: 35514503
- PMCID: PMC9067298
- DOI: 10.1039/c9ra03905h
Linker length in fluorophore-cholesterol conjugates directs phase selectivity and cellular localisation in GUVs and live cells
Abstract
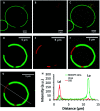
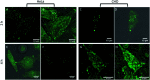
Lipid membrane fluorescent probes that are both domain-selective and compatible with demanding microscopy methods are crucial to elucidate the presence and function of rafts and domains in cells and biophysical models. Whereas targeting fluorescent probes to liquid-disordered (Ld) domains is relatively facile, it is far more difficult to direct probes with high selectivity to liquid-ordered (Lo) domains. Here, a simple, one-pot approach to probe-cholesterol conjugation is described using Steglich esterification to synthesise two identical BODIPY derivatives that differ only in the length of the aliphatic chain between the dye and cholesterol. In the first, BODIPY-Ar-Chol, the probe and cholesterol were directly ester linked and in the second BODIPY-Ahx-Chol, a hexyl linker separated probe from cholesterol. Uptake and distribution of each probe was compared in ternary, phase separated giant unilamellar vesicles (GUVs) using a commercial Ld marker as a reference. BODIPY-Ar-Chol targets almost exclusively the Ld domains with selectivity of >90% whereas by contrast introducing the C6 linker between the probe and cholesterol drove the probe to Lo with excellent selectivity (>80%). The profound impact of the linker length extended also to uptake and distribution in live mammalian cells. BODIPY-Ahx-Chol associates strongly with the plasma membrane where it partitioned preferably into opposing micron dimensioned do-mains to a commercial Ld marker and its concentration at the membrane was reduced by cyclodextrin treatment of the cells. By contrast the BODIPY-Ahx-Chol permeated the membrane and localised strongly to lipid droplets within the cell. The data demonstrates the profound influence of linker length in cholesterol bioconjugates in directing the probe.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Membrane fluidity and lipid order in ternary giant unilamellar vesicles using a new bodipy-cholesterol derivative.Biophys J. 2009 Apr 8;96(7):2696-708. doi: 10.1016/j.bpj.2008.12.3922. Biophys J. 2009. PMID: 19348752 Free PMC article.
-
Membrane orientation and lateral diffusion of BODIPY-cholesterol as a function of probe structure.Biophys J. 2013 Nov 5;105(9):2082-92. doi: 10.1016/j.bpj.2013.09.031. Biophys J. 2013. PMID: 24209853 Free PMC article.
-
Shape changes and vesicle fission of giant unilamellar vesicles of liquid-ordered phase membrane induced by lysophosphatidylcholine.Langmuir. 2004 Oct 26;20(22):9526-34. doi: 10.1021/la049481g. Langmuir. 2004. PMID: 15491182
-
Exploring the raft-hypothesis by probing planar bilayer patches of free-standing giant vesicles at nanoscale resolution, with and without Na,K-ATPase.Biochim Biophys Acta. 2016 Dec;1858(12):3041-3049. doi: 10.1016/j.bbamem.2016.09.001. Epub 2016 Sep 9. Biochim Biophys Acta. 2016. PMID: 27616046 Review.
-
Amyloid-β Interactions with Lipid Rafts in Biomimetic Systems: A Review of Laboratory Methods.Methods Mol Biol. 2021;2187:47-86. doi: 10.1007/978-1-0716-0814-2_4. Methods Mol Biol. 2021. PMID: 32770501 Review.
Cited by
-
Rhenium(I) conjugates as tools for tracking cholesterol in cells.Metallomics. 2022 Aug 2;14(8):mfac040. doi: 10.1093/mtomcs/mfac040. Metallomics. 2022. PMID: 35657681 Free PMC article.
-
BODIPY Conjugates as Functional Compounds for Medical Diagnostics and Treatment.Molecules. 2022 Feb 18;27(4):1396. doi: 10.3390/molecules27041396. Molecules. 2022. PMID: 35209191 Free PMC article. Review.
-
Influence of fluorophore and linker length on the localization and trafficking of fluorescent sterol probes.Sci Rep. 2020 Dec 16;10(1):22053. doi: 10.1038/s41598-020-78085-9. Sci Rep. 2020. PMID: 33328481 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

