Preimplantation chromosomal mosaics, chimaeras and confined placental mosaicism
- PMID: 35514539
- PMCID: PMC9066951
- DOI: 10.1530/RAF-21-0095
Preimplantation chromosomal mosaics, chimaeras and confined placental mosaicism
Abstract
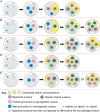
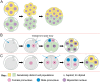
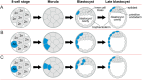
Some human preimplantation embryos are chromosomally mosaic. For technical reasons, estimates of the overall frequency vary widely from <15 to >90% and the true frequency remains unknown. Aneuploid/diploid and aneuploid/aneuploid mosaics typically arise during early cleavage stages before the embryonic genome is fully activated and when cell cycle checkpoints are not operating normally. Other mosaics include chaotic aneuploid mosaics and mixoploids, some of which arise by abnormal chromosome segregation at the first cleavage division. Chimaeras are similar to mosaics, in having two genetically distinct cell populations, but they arise from more than one zygote and occur less often. After implantation, the frequency of mosaic embryos declines to about 2% and most are trisomic/diploid mosaics, with trisomic cells confined to the placenta. Thus, few babies are born with chromosomal mosaicism. This review discusses the origin of different types of chromosomal mosaics and chimaeras; their fate and the relationship between preimplantation chromosomal mosaicism and confined placental mosaicism in human conceptuses and animal models. Abnormal cells in mosaic embryos may be depleted by cell death, other types of cell selection or cell correction but the most severely affected mosaic embryos probably die. Trisomic cells could become restricted to placental lineages if cell selection or correction is less effective in placental lineages and/or they are preferentially allocated to a placental lineage. However, the relationship between preimplantation mosaicism and confined placental mosaicism may be complex because the specific chromosome(s) involved will influence whether chromosomally abnormal cells survive predominately in the placental trophoblast and/or placental mesenchyme.
Lay summary: Human cells normally have 23 pairs of chromosomes, which carry the genes. During the first few days of development, some human embryos are chromosomal mosaics. These mosaic embryos have both normal cells and cells with an abnormal number of chromosomes, which arise from the same fertilised egg. (More rarely, the different cell populations arise from more than one fertilised egg and these embryos are called chimaeras.) If chromosomally abnormal cells survive to term, they could cause birth defects. However, few abnormal cells survive and those that do are usually confined to the placenta, where they are less likely to cause harm. It is not yet understood how this restriction occurs but the type of chromosomal abnormality influences which placental tissues are affected. This review discusses the origin of different types of chromosomally abnormal cells, their fate and how they might become confined to the placenta in humans and animal models.
Keywords: aneuploidy; chimaera; chromosomal mosaic; confined placental mosaicism; mixoploidy.
© The authors.
Figures

Similar articles
-
The human embryonic genome is karyotypically complex, with chromosomally abnormal cells preferentially located away from the developing fetus.Hum Reprod. 2023 Jan 5;38(1):180-188. doi: 10.1093/humrep/deac238. Hum Reprod. 2023. PMID: 36350568 Free PMC article.
-
The use of copy number loads to designate mosaicism in blastocyst stage PGT-A cycles: fewer is better.Hum Reprod. 2023 May 2;38(5):982-991. doi: 10.1093/humrep/dead049. Hum Reprod. 2023. PMID: 36928183
-
Healthy Live Births after the Transfer of Mosaic Embryos: Self-Correction or PGT-A Overestimation?Genes (Basel). 2023 Dec 21;15(1):18. doi: 10.3390/genes15010018. Genes (Basel). 2023. PMID: 38275600 Free PMC article. Review.
-
Genotypically unbalanced diploid<==>diploid foetal mouse chimaeras: possible relevance to human confined mosaicism.Genet Res. 1994 Apr;63(2):87-99. doi: 10.1017/s0016672300032195. Genet Res. 1994. PMID: 8026741
-
The cytogenetic constitution of human blastocysts: insights from comprehensive chromosome screening strategies.Hum Reprod Update. 2019 Jan 1;25(1):15-33. doi: 10.1093/humupd/dmy036. Hum Reprod Update. 2019. PMID: 30395265 Review.
Cited by
-
Reproductive genetics at a crossroads: the challenges posed by hominid pregnancy loss.J Assist Reprod Genet. 2024 May;41(5):1125-1126. doi: 10.1007/s10815-024-03142-5. J Assist Reprod Genet. 2024. PMID: 38748359 Free PMC article. No abstract available.
-
Sperm intrusion into the implantation-stage blastocyst and its potential biological significance.Evol Med Public Health. 2023 Dec 23;12(1):1-6. doi: 10.1093/emph/eoad043. eCollection 2024. Evol Med Public Health. 2023. PMID: 38234421 Free PMC article.
-
Discrepancy between non-invasive prenatal testing result and fetal karyotype caused by rare confined placental mosaicism: A case report.World J Clin Cases. 2022 Aug 26;10(24):8641-8647. doi: 10.12998/wjcc.v10.i24.8641. World J Clin Cases. 2022. PMID: 36157819 Free PMC article.
-
Sometimes karyotype resolves the case!Front Genet. 2024 Feb 28;15:1371166. doi: 10.3389/fgene.2024.1371166. eCollection 2024. Front Genet. 2024. PMID: 38482384 Free PMC article. No abstract available.
References
-
- Anderson D, Billingham RE, Lampkin GH, Medawar PB.1951The use of skin grafting to distinguish between monozygotic and dizygotic twins in cattle. Heredity 5379–397. (10.1038/hdy.1951.38) - DOI
-
- Azuma S, Fukuda Y, Toyoda Y.1991aStudies on the production of 2n↔3n chimeric mouse embryos. II. Post-implantation development. Japanese Journal of Animal Reproduction 3779–87 (in Japanese). (10.1262/jrd1977.37.79) - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

