Synthesis of isobemisiose, neosartose, and fischerose: three α-1,6-linked trehalose-based oligosaccharides identified from Neosartorya fischeri
- PMID: 35514568
- PMCID: PMC9054618
- DOI: 10.1039/d0ra04137h
Synthesis of isobemisiose, neosartose, and fischerose: three α-1,6-linked trehalose-based oligosaccharides identified from Neosartorya fischeri
Abstract
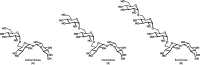
Three complex α-1,6-linked trehalose-based oligosaccharides with unique preservation properties, isobemisiose, neosartose, and fischerose, were recently identified from the extreme stress-tolerant ascospores of Neosartorya fischeri. Herein, we report the first concise, scalable, and iterative chemical synthesis of these oligosaccharides from a differentially protected thioglycoside donor and a selectively protected, asymmetric trehalose acceptor. This work constitutes an improved synthesis of isobemisiose, and is also the first reported synthesis of neosartose, a tetrasaccharide, and fischerose, a pentasaccharide, in good yield. Importantly, in-depth studies of biological function are enabled by this synthetic platform.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures



References
-
- Kastrinsky D. B., McBridge N. S., Backus K. M., LeBlanc J. J. and Barry III C. E., Mycolic Acid/Cyclopropane Fatty Acid/Fatty Acid Biosynthesis and Health Relations, in Comprehensive Natural Products II, ed. H.-W. Liu and L. Mander, Elsevier, 2010, vol. 1.04, pp. 65–145
-
- Higashiyama T. Pure Appl. Chem. 2002;74:1263.
LinkOut - more resources
Full Text Sources

