Study of M(iii)-cyclam (M = Rh, Ru; cyclam = 1,4,8,11-tetraazacyclotetradecane) complexes as novel methanol resistant electrocatalysts for the oxygen reduction reaction
- PMID: 35514573
- PMCID: PMC9054714
- DOI: 10.1039/d0ra02904a
Study of M(iii)-cyclam (M = Rh, Ru; cyclam = 1,4,8,11-tetraazacyclotetradecane) complexes as novel methanol resistant electrocatalysts for the oxygen reduction reaction
Abstract
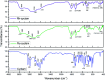
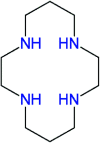
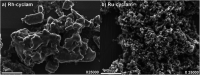
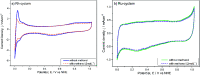
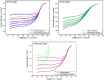
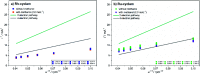
Transition metal macrocyclic complexes have acquired relevance as electrocatalysts to perform oxygen electroreduction in acid media as an alternative to platinum. This work presents two macrocyclic complexes using cyclam as ligand, which has a much simpler molecular structure (smaller size, no π-electrons, etc.) than the well studied porphyrins and phthalocyanines as transition metal complexes. Such compounds are usually subjected to thermal treatments at relatively high temperatures (800-900 °C) which result in ligand decomposition, leaving the so-called MN x active sites. In contrast, the complexes reported in this work are efficient electrocatalysts for the oxygen reduction reaction (ORR) in their original molecular structure, with no thermal treatments of any kind applied. The electrocatalytic activity of the Rh(iii)-cyclam and Ru(iii)-cyclam complexes during the ORR in the absence and presence of methanol (2 mol L-1) was evaluated by voltammetry techniques. The kinetic parameters of the novel materials for the reaction were determined. The exchange current density (j 0) values, directly related to the charge transfer velocity, are of the same order as or higher than those of platinum/Vulcan® nanoparticles. In addition, they are practically unaffected by methanol, therefore, becoming interesting candidates to be evaluated as cathodes in polymer electrolyte membrane and direct methanol fuel cells.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

Similar articles
-
Synthesis of cis and trans bis-alkynyl complexes of Cr(III) and Rh(III) supported by a tetradentate macrocyclic amine: a spectroscopic investigation of the M(III)-alkynyl interaction.Inorg Chem. 2011 Oct 3;50(19):9354-64. doi: 10.1021/ic2009336. Epub 2011 Sep 2. Inorg Chem. 2011. PMID: 21888339
-
Sustainable metal alkynyl chemistry: 3d metals and polyaza macrocyclic ligands.Chem Commun (Camb). 2016 Feb 25;52(16):3271-9. doi: 10.1039/c5cc09365a. Chem Commun (Camb). 2016. PMID: 26779578
-
Electronic structures of [Ru(II)(cyclam)(Et(2)dtc)](+), [Ru(cyclam)(tdt)](+), and [Ru(cyclam)(tdt)](2+): an X-ray absorption spectroscopic and computational study (tdt = toluene-3,4-dithiolate; Et(2)dtc = N,N-diethyldithiocarbamate(1-)).Inorg Chem. 2009 Oct 19;48(20):9754-66. doi: 10.1021/ic9011845. Inorg Chem. 2009. PMID: 19769378
-
Bioinspired Transition-Metal Complexes as Electrocatalysts for the Oxygen Reduction Reaction.Chemistry. 2019 Mar 12;25(15):3726-3739. doi: 10.1002/chem.201803764. Epub 2018 Dec 21. Chemistry. 2019. PMID: 30203875 Review.
-
Platinum-based oxygen reduction electrocatalysts.Acc Chem Res. 2013 Aug 20;46(8):1848-57. doi: 10.1021/ar300359w. Epub 2013 Jun 28. Acc Chem Res. 2013. PMID: 23808919 Review.
Cited by
-
RuIII(edta) complexes as molecular redox catalysts in chemical and electrochemical reduction of dioxygen and hydrogen peroxide: inner-sphere versus outer-sphere mechanism.RSC Adv. 2021 Jun 16;11(35):21359-21366. doi: 10.1039/d1ra03293c. eCollection 2021 Jun 15. RSC Adv. 2021. PMID: 35478799 Free PMC article.
-
Advances in the use of metal-free tetrapyrrolic macrocycles as catalysts.Beilstein J Org Chem. 2024 Nov 27;20:3085-3112. doi: 10.3762/bjoc.20.257. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 39624654 Free PMC article. Review.
-
Advances in the synthesis and applications of macrocyclic polyamines.R Soc Open Sci. 2024 Jun 19;11(6):231979. doi: 10.1098/rsos.231979. eCollection 2024 Jun. R Soc Open Sci. 2024. PMID: 39092147 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

