Parameter optimization and degradation mechanism for electrocatalytic degradation of 2,4-diclorophenoxyacetic acid (2,4-D) herbicide by lead dioxide electrodes
- PMID: 35514628
- PMCID: PMC9060676
- DOI: 10.1039/c8ra10105a
Parameter optimization and degradation mechanism for electrocatalytic degradation of 2,4-diclorophenoxyacetic acid (2,4-D) herbicide by lead dioxide electrodes
Abstract
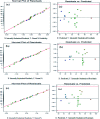
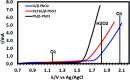
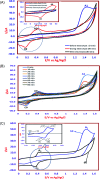
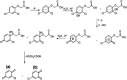
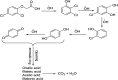
2,4-Dichlorophenoxyacetic acid (2,4-D) is one of the most commonly used herbicides in the world. In this work, the electro-catalytic degradation of 2,4-D herbicide from aqueous solutions was evaluated using three anode electrodes, i.e., lead dioxide coated on stainless steel 316 (SS316/β-PbO2), lead dioxide coated on a lead bed (Pb/β-PbO2), and lead dioxide coated on graphite (G/β-PbO2). The structure and morphology of the prepared electrodes were studied by X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX). The process of herbicide degradation was monitored during constant current electrolysis using cyclic voltammetry (CV). In this study, the experiments were designed based on the central composite design (CCD) and were analyzed and modeled by response surface methodology (RSM) to demonstrate the operational variables and the interactive effect of three independent variables on 3 responses. The effects of parameters including pH (3-11), current density (j = 1-5 mA cm-2) and electrolysis time (20-80 min) were studied. The results showed that, at j = 5 mA cm-2, by increasing the reaction time from 20 to 80 min and decreasing the pH from 11 to 3, the 2,4-D herbicide degradation efficiency using SS316/β-PbO2, Pb/β-PbO2 and G/β-PbO2 anode electrodes was observed to be 60.4, 75.9 and 89.8%, respectively. Moreover, the results showed that the highest COD and TOC removal efficiencies using the G/β-PbO2 electrode were 83.7 and 78.5%, under the conditions pH = 3, electrolysis time = 80 min and j = 5 mA cm-2, respectively. It was also found that G/β-PbO2 has lower energy consumption (EC) (5.67 kW h m-3) compared to the two other studied electrodes (SS316/β-PbO2 and Pb/β-PbO2). The results showed a good correlation between the experimental values and the predicted values of the quadratic model (P < 0.05). Results revealed that the electrochemical process using the G/β-PbO2 anode electrode has an acceptable efficiency in the degradation of 2,4-D herbicide and can be used as a proper pretreatment technique to treat wastewater containing resistant pollutants, e.g., phenoxy group herbicides (2,4-D).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Samarghandi M. R. Mohammadi M. Karami A. Tabandeh L. Dargahi A. Amirian F. Pol. J. Environ. Stud. 2017;26:2189–2195. doi: 10.15244/pjoes/70387. - DOI
-
- Arias-Estévez M. López-Periago E. Martínez-Carballo E. Simal-Gándara J. Mejuto J.-C. García-Río L. Agric., Ecosyst. Environ. 2008;123:247–260. doi: 10.1016/j.agee.2007.07.011. - DOI
-
- Chidambaram R. Ecol. Eng. 2016;92:97–105. doi: 10.1016/j.ecoleng.2016.03.020. - DOI
LinkOut - more resources
Full Text Sources

