Poly(ethylene oxide)/Ag ions and nanoparticles/1-hexyl-3-methylimidazolium tetrafluoroborate composite membranes with long-term stability for olefin/paraffin separation
- PMID: 35514652
- PMCID: PMC9060575
- DOI: 10.1039/c8ra09274e
Poly(ethylene oxide)/Ag ions and nanoparticles/1-hexyl-3-methylimidazolium tetrafluoroborate composite membranes with long-term stability for olefin/paraffin separation
Abstract
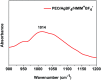
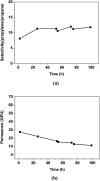
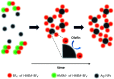
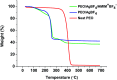
A poly(ethylene oxide)(PEO)/AgBF4/1-hexyl-3-methylimidazolium tetrafluoroborate (HMIM+BF4 -) composite membrane that exhibits long-term stability was prepared for olefin/paraffin separation. The membrane was prepared by simply adding AgBF4 and HMIM+BF4 - to a solution of PEO. Long-term stability testing showed that the separation performance of the membrane is maintained for ≈100 h owing to the Ag NPs formed in the membrane, which are olefin carriers, being stabilized by HMIM+BF4 -. In terms of separation performance, the PEO/AgBF4/HMIM+BF4 - composite membrane exhibited a propylene/propane selectivity of 11.8 and a mixed-gas permeance of 11.3 GPU. We also investigated the factors that determine separation performance by comparison with a PEO/AgBF4/1-butyl-3-methylimidazolium tetrafluoroborate (BMIM+BF4 -) composite membrane. The PEO/AgBF4/HMIM+BF4 - composite membrane was characterized by scanning electron microscopy, FT-IR spectroscopy, ultraviolet-visible spectroscopy, thermogravimetric analysis, and Raman spectroscopy.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Park C. H. Lee J. H. Jung J. P. Kim J. H. J. Membr. Sci. 2017;533:48–56. doi: 10.1016/j.memsci.2017.03.023. - DOI
-
- Koros W. J. AIChE J. 2004;50:2326–2334. doi: 10.1002/aic.10330. - DOI
-
- Jeong S. Kang S. W. Chem. Eng. J. 2017;327:500–504. doi: 10.1016/j.cej.2017.06.117. - DOI
-
- True W. R. Oil Gas J. 2012;110:78–84.
-
- Eldridge R. B. Ind. Eng. Chem. Res. 1993;32:2208–2212. doi: 10.1021/ie00022a002. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

