Broadening the scope of sortagging
- PMID: 35514663
- PMCID: PMC9060782
- DOI: 10.1039/c8ra06705h
Broadening the scope of sortagging
Abstract
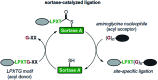
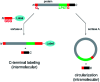
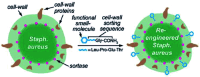
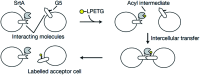
Sortases are enzymes occurring in the cell wall of Gram-positive bacteria. Sortase A (SrtA), the best studied sortase class, plays a key role in anchoring surface proteins with the recognition sequence LPXTG covalently to oligoglycine units of the bacterial cell wall. This unique transpeptidase activity renders SrtA attractive for various purposes and motivated researchers to study multiple in vivo and in vitro ligations in the last decades. This ligation technique is known as sortase-mediated ligation (SML) or sortagging and developed to a frequently used method in basic research. The advantages are manifold: extremely high substrate specificity, simple access to substrates and enzyme, robust nature and easy handling of sortase A. In addition to the ligation of two proteins or peptides, early studies already included at least one artificial (peptide equipped) substrate into sortagging reactions - which demonstrates the versatility and broad applicability of SML. Thus, SML is not only a biology-related technique, but has found prominence as a major interdisciplinary research tool. In this review, we provide an overview about the use of sortase A in interdisciplinary research, mainly for protein modification, synthesis of protein-polymer conjugates and immobilization of proteins on surfaces.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures















Similar articles
-
Structural and biochemical analyses of selectivity determinants in chimeric Streptococcus Class A sortase enzymes.Protein Sci. 2022 Mar;31(3):701-715. doi: 10.1002/pro.4266. Epub 2022 Jan 3. Protein Sci. 2022. PMID: 34939250 Free PMC article.
-
Structure and specificity of a new class of Ca2+-independent housekeeping sortase from Streptomyces avermitilis provide insights into its non-canonical substrate preference.J Biol Chem. 2017 Apr 28;292(17):7244-7257. doi: 10.1074/jbc.M117.782037. Epub 2017 Mar 7. J Biol Chem. 2017. PMID: 28270507 Free PMC article.
-
Sequence variation in the β7-β8 loop of bacterial class A sortase enzymes alters substrate selectivity.J Biol Chem. 2021 Aug;297(2):100981. doi: 10.1016/j.jbc.2021.100981. Epub 2021 Jul 22. J Biol Chem. 2021. PMID: 34302812 Free PMC article.
-
Sortase A: A chemoenzymatic approach for the labeling of cell surfaces.Biotechnol Bioeng. 2021 Dec;118(12):4577-4589. doi: 10.1002/bit.27935. Epub 2021 Sep 27. Biotechnol Bioeng. 2021. PMID: 34491580 Review.
-
Anchoring of LPXTG-Like Proteins to the Gram-Positive Cell Wall Envelope.Curr Top Microbiol Immunol. 2017;404:159-175. doi: 10.1007/82_2016_8. Curr Top Microbiol Immunol. 2017. PMID: 27097813 Review.
Cited by
-
Engineered peptide ligases for cell signaling and bioconjugation.Biochem Soc Trans. 2020 Jun 30;48(3):1153-1165. doi: 10.1042/BST20200001. Biochem Soc Trans. 2020. PMID: 32539119 Free PMC article. Review.
-
Protein scaffolds: A tool for multi-enzyme assembly.Biotechnol Rep (Amst). 2021 Sep 6;32:e00670. doi: 10.1016/j.btre.2021.e00670. eCollection 2021 Dec. Biotechnol Rep (Amst). 2021. PMID: 34824995 Free PMC article. Review.
-
Approaches for peptide and protein cyclisation.Org Biomol Chem. 2021 May 12;19(18):3983-4001. doi: 10.1039/d1ob00411e. Org Biomol Chem. 2021. PMID: 33978044 Free PMC article. Review.
-
Strategies for Site-Specific Labeling of Receptor Proteins on the Surfaces of Living Cells by Using Genetically Encoded Peptide Tags.Chembiochem. 2021 May 14;22(10):1717-1732. doi: 10.1002/cbic.202000797. Epub 2021 Feb 26. Chembiochem. 2021. PMID: 33428317 Free PMC article. Review.
-
Functionalized High Mannose-Specific Lectins for the Discovery of Type I Mannosidase Inhibitors.Angew Chem Int Ed Engl. 2021 May 25;60(22):12313-12318. doi: 10.1002/anie.202101249. Epub 2021 Apr 26. Angew Chem Int Ed Engl. 2021. PMID: 33728787 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

