Fabrication of Ag/AgBr/Ag3VO4 composites with high visible light photocatalytic performance
- PMID: 35514669
- PMCID: PMC9060689
- DOI: 10.1039/c8ra10538c
Fabrication of Ag/AgBr/Ag3VO4 composites with high visible light photocatalytic performance
Abstract
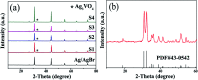
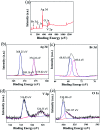
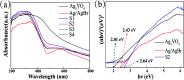
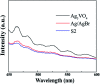
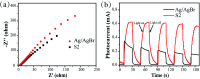
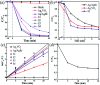
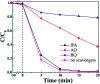
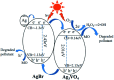
Herein, Ag/AgBr/Ag3VO4 composites were synthesized by a simple continuous precipitation method. The obtained composites were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive spectrometry (EDS), X-ray photoelectron spectroscopy (XPS), UV-vis diffuse reflectance spectroscopy and photoluminescence spectroscopy (PL). Photocatalytic performance of the composites was assessed by the degradation of methyl orange (MO) and tetracycline hydrochloride (TC) under visible light, and the effects of different nominal mass ratios of AgBr and Ag3VO4 on the photocatalytic activity were investigated. The results showed that after 20 min of visible light irradiation (λ > 420 nm), the removal rate of MO in the presence of a 5 : 1 sample reached 98.6%. The EIS and photocurrent results demonstrated that the enhancement of the visible light photocatalytic activity was attributed to the efficient electron-hole pair separation. In addition, the scavenging reactions conducted via the addition of different scavengers confirmed that h+ and ·O2- were the main active species in the reaction. The present study offers potential for the degradation of contaminants.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
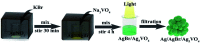


Similar articles
-
Visible light photocatalytic activity enhancement and mechanism of AgBr/Ag3PO4 hybrids for degradation of methyl orange.J Hazard Mater. 2012 May 30;217-218:107-15. doi: 10.1016/j.jhazmat.2012.03.002. Epub 2012 Mar 7. J Hazard Mater. 2012. PMID: 22464754
-
Double Effect Electron Transfer System in the AgBr/ZnO Composite with Enhanced Photocatalytic Degradation Performance against 3-Chlorophenol under Visible Light Irradiation.Photochem Photobiol. 2018 Nov;94(6):1225-1233. doi: 10.1111/php.12977. Epub 2018 Aug 8. Photochem Photobiol. 2018. PMID: 29981152
-
Ag-Ag3VO4/AgIO3 composites with enhanced visible-light-driven catalytic activity.J Colloid Interface Sci. 2018 Aug 15;524:16-24. doi: 10.1016/j.jcis.2018.04.001. Epub 2018 Apr 3. J Colloid Interface Sci. 2018. PMID: 29627668
-
A novel visible-light-response plasmonic photocatalyst CNT/Ag/AgBr and its photocatalytic properties.Phys Chem Chem Phys. 2013 Apr 28;15(16):5821-30. doi: 10.1039/c3cp44104k. Epub 2013 Mar 13. Phys Chem Chem Phys. 2013. PMID: 23487033
-
Fabrication of Ag2O/Ag decorated ZnAl-layered double hydroxide with enhanced visible light photocatalytic activity for tetracycline degradation.Ecotoxicol Environ Saf. 2019 May 15;172:423-431. doi: 10.1016/j.ecoenv.2019.01.080. Epub 2019 Feb 5. Ecotoxicol Environ Saf. 2019. PMID: 30735974
Cited by
-
Strong Intermixing Effects of LFO1-x/STOx toward the Development of Efficient Photoanodes for Photoelectrocatalytic Applications.Nanomaterials (Basel). 2023 Oct 29;13(21):2863. doi: 10.3390/nano13212863. Nanomaterials (Basel). 2023. PMID: 37947708 Free PMC article.
-
Immobilized Ag3PO4/GO on 3D nickel foam and its photocatalytic degradation of norfloxacin antibiotic under visible light.RSC Adv. 2020 Jan 27;10(8):4427-4435. doi: 10.1039/c9ra08678a. eCollection 2020 Jan 24. RSC Adv. 2020. PMID: 35495222 Free PMC article.
-
Photocatalytic Degradation of Some Typical Antibiotics: Recent Advances and Future Outlooks.Int J Mol Sci. 2022 Jul 24;23(15):8130. doi: 10.3390/ijms23158130. Int J Mol Sci. 2022. PMID: 35897716 Free PMC article. Review.
-
Tuning Ni-MoO2 Catalyst-Ionomer and Electrolyte Interaction for Water Electrolyzers with Anion Exchange Membranes.ACS Appl Energy Mater. 2021 Apr 26;4(4):3327-3340. doi: 10.1021/acsaem.0c03072. Epub 2021 Mar 23. ACS Appl Energy Mater. 2021. PMID: 34056552 Free PMC article.
-
Transformation of novel TiOF2 nanoparticles to cluster TiO2-{001/101} and its degradation of tetracycline hydrochloride under simulated sunlight.RSC Adv. 2020 Nov 24;10(70):42860-42873. doi: 10.1039/d0ra08476j. eCollection 2020 Nov 23. RSC Adv. 2020. PMID: 35514916 Free PMC article.
References
-
- Jo W.-K. Tayade R. J. Chin. J. Catal. 2014;35:1781–1792. doi: 10.1016/S1872-2067(14)60205-9. - DOI
-
- Rai H. S. Bhattacharyya M. S. Singh J. Bansal T. K. Vats P. Banerjee U. C. Crit. Rev. Env. Sci. Technol. 2005;35:219–238. doi: 10.1080/10643380590917932. - DOI
-
- Hu J. Zhang P. An W. Liu L. Liang Y. Cui W. Appl. Catal., B. 2019;245:130–142. doi: 10.1016/j.apcatb.2018.12.029. - DOI
LinkOut - more resources
Full Text Sources

