Structure and photocatalytic performance of rice husk-like Ba-doped GaOOH under light irradiation
- PMID: 35514685
- PMCID: PMC9065475
- DOI: 10.1039/c9ra03670a
Structure and photocatalytic performance of rice husk-like Ba-doped GaOOH under light irradiation
Abstract
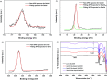
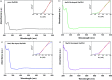
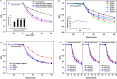
The effects of Ba-doping on the structure and photocatalytic performance of GaOOH were investigated for the first time in this paper. XRD, SEM, TEM, XPS, UPS, FT-IR, UV-Vis DRS, PL, BET and EPR characterizations were carried out to analyze the properties of Ba-doped GaOOH. The results showed that GaOOH crystallized well with the orthorhombic crystal system with space group Pbnm. The lattice parameters of GaOOH were found to be a = 4.509526 Å, b = 9.771034 Å and c = 2.969284 Å. The transition in the structural morphology of GaOOH before and after Ba-doping was observed in SEM pictures in which the morphology of GaOOH varied from wood-like to rice husk-like. At the same time, the specific surface area of 4 wt% Ba-doped GaOOH (21.5854 m2 g-1) was 3.42 times that of pure GaOOH (6.3047 m2 g-1). Ba-doping caused a red shift of the band gap according to UV-Vis DRS results. The enhanced defect states caused by Ba-doping was confirmed by PL results, which decreased the recombination rate of photogenerated electrons and photogenerated holes. Compared with pure GaOOH, when GaOOH with different Ba content was used as photocatalyst, the removal rate of enrofloxacin was increased by more than 20% only by illumination for 60 min. In addition, Ba-doped GaOOH had excellent stability and could be reused, which could reduce costs and increase the potential of its practical application.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Synthesis of a GaOOH/ZnBiTaO5 heterojunction photocatalyst with enhanced photocatalytic performance toward enrofloxacin.RSC Adv. 2020 Jan 27;10(8):4286-4292. doi: 10.1039/c9ra09741d. eCollection 2020 Jan 24. RSC Adv. 2020. PMID: 35495278 Free PMC article.
-
Dispersed GaOOH rods loaded on the surface of ZnBiNbO5 particles with enhanced photocatalytic activity toward enrofloxacin.RSC Adv. 2019 Oct 9;9(55):32027-32033. doi: 10.1039/c9ra06153c. eCollection 2019 Oct 7. RSC Adv. 2019. PMID: 35530812 Free PMC article.
-
Enhanced photocatalytic activity, transport properties and electronic structure of Mn doped GdFeO3 synthesized using the sol-gel process.Phys Chem Chem Phys. 2021 Aug 4;23(30):16060-16076. doi: 10.1039/d1cp00621e. Phys Chem Chem Phys. 2021. PMID: 34291256
-
Synthesis of Tin-Doped Three-Dimensional Flower-like Bismuth Tungstate with Enhanced Photocatalytic Activity.Int J Mol Sci. 2022 Jul 29;23(15):8422. doi: 10.3390/ijms23158422. Int J Mol Sci. 2022. PMID: 35955557 Free PMC article.
-
Preparation and Characterization of Mo Doped in BiVO₄ with Enhanced Photocatalytic Properties.Materials (Basel). 2017 Aug 21;10(8):976. doi: 10.3390/ma10080976. Materials (Basel). 2017. PMID: 28825676 Free PMC article.
Cited by
-
Synthesis of a GaOOH/ZnBiTaO5 heterojunction photocatalyst with enhanced photocatalytic performance toward enrofloxacin.RSC Adv. 2020 Jan 27;10(8):4286-4292. doi: 10.1039/c9ra09741d. eCollection 2020 Jan 24. RSC Adv. 2020. PMID: 35495278 Free PMC article.
-
Dispersed GaOOH rods loaded on the surface of ZnBiNbO5 particles with enhanced photocatalytic activity toward enrofloxacin.RSC Adv. 2019 Oct 9;9(55):32027-32033. doi: 10.1039/c9ra06153c. eCollection 2019 Oct 7. RSC Adv. 2019. PMID: 35530812 Free PMC article.
References
-
- Qian H. S. Gunawan P. Zhang Y. X. Lin G. F. Zheng J. W. Xu R. Cryst. Growth Des. 2008;8:1282. doi: 10.1021/cg701004w. - DOI
-
- Huang C. C. Yeh C. S. New J. Chem. 2010;34:103. doi: 10.1039/B9NJ00392D. - DOI
-
- Shi L. H. Zhang J. Wu S. Li Y. A. Jiang L. N. Cui Q. L. J. Am. Ceram. Soc. 2014;97:2607–2614. doi: 10.1111/jace.12953. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

