Coated silver nanoparticles: synthesis, cytotoxicity, and optical properties
- PMID: 35514687
- PMCID: PMC9065456
- DOI: 10.1039/c9ra02907a
Coated silver nanoparticles: synthesis, cytotoxicity, and optical properties
Abstract
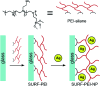
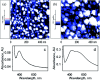
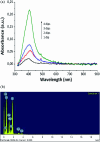
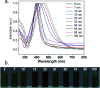
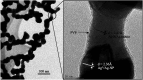
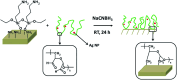
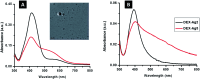
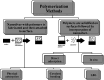
Coated silver nanoparticles (AgNPs) have recently become a topic of interest due to the fact that they have several applications such as in electronic, antimicrobial, industrial, optical, and medical fields as biosensors and drug delivery systems. However, the use of AgNPs in medical fields remains somewhat limited due to their probable cytotoxic effect. Researchers have succeeded in reducing the toxicity of silver particles by coating them with different substances. Generally, the coating of AgNPs leads to change in their properties depending on the type of the coating material as well as the layer thickness. This review covers the state-of-the-art technologies behind (a) the synthesis of coated AgNPs including coating methods and coating materials, (b) the cytotoxicity of coated AgNPs and (c) the optical properties of coated AgNPs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures













References
-
- Guerra F. D. Campbell M. L. Whitehead D. C. Alexis F. ChemistrySelect. 2017a;2:9889–9894.
-
- Guerra F. D. Smith G. D. Alexis F. Whitehead D. C. Aerosol Air Qual. Res. 2017b;17:209–217.
Publication types
LinkOut - more resources
Full Text Sources

