Immobilization of Lewis acidic ionic liquid on perlite nanoparticle surfaces as a highly efficient solid acid catalyst for the solvent-free synthesis of xanthene derivatives
- PMID: 35514727
- PMCID: PMC9065349
- DOI: 10.1039/c9ra03312b
Immobilization of Lewis acidic ionic liquid on perlite nanoparticle surfaces as a highly efficient solid acid catalyst for the solvent-free synthesis of xanthene derivatives
Abstract
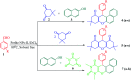
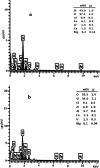
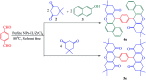
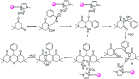
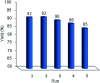
In this study, perlite nanoparticles were prepared through a simple method and then modified with Lewis acidic ionic liquid (perlite NP@IL/ZrCl4) through a two step procedure. The prepared solid acid catalyst was characterized by Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDX) and thermo gravimetric analysis (TGA). Perlite NP@IL/ZrCl4 was used as a new solid acid, reusable and green heterogeneous nanocatalyst for the one-pot synthesis of xanthene derivatives. Synthesis of xanthenes was performed under solvent free conditions using a catalytic amount (0.005 g, 0.4 mol%) of the prepared catalyst with simple work-up and high to excellent yield of products. The reusability and high efficiency of this catalyst makes this method attractive for large scale environment-friendly operations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

