BREEZE: Open-label clinical study to evaluate the safety and tolerability of treprostinil inhalation powder as Tyvaso DPI™ in patients with pulmonary arterial hypertension
- PMID: 35514770
- PMCID: PMC9063953
- DOI: 10.1002/pul2.12063
BREEZE: Open-label clinical study to evaluate the safety and tolerability of treprostinil inhalation powder as Tyvaso DPI™ in patients with pulmonary arterial hypertension
Abstract
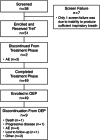
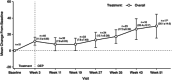
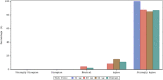
Inhaled treprostinil is an approved therapy for pulmonary arterial hypertension (PAH) and pulmonary hypertension associated with interstitial lung disease in the United States. Studies have confirmed the robust benefits and safety of nebulized inhaled treprostinil, but it requires a time investment for nebulizer preparation, maintenance, and treatment. A small, portable treprostinil dry powder inhaler has been developed for the treatment of PAH. The primary objective of this study was to evaluate the safety and tolerability of treprostinil inhalation powder (TreT) in patients currently treated with treprostinil inhalation solution. Fifty-one patients on a stable dose of treprostinil inhalation solution enrolled and transitioned to TreT at a corresponding dose. Six-minute walk distance (6MWD), device preference and satisfaction (Preference Questionnaire for Inhaled Treprostinil Devices [PQ-ITD]), PAH Symptoms and Impact (PAH-SYMPACT®) questionnaire, and systemic exposure and pharmacokinetics for up to 5 h were assessed at baseline for treprostinil inhalation solution and at Week 3 for TreT. Adverse events (AEs) were consistent with studies of inhaled treprostinil in patients with PAH, and there were no study drug-related serious AEs. Statistically significant improvements occurred in 6MWD, PQ-ITD, and PAH-SYMPACT. Forty-nine patients completed the 3-week treatment phase and all elected to participate in an optional extension phase. These results demonstrate that, in patients with PAH, transition from treprostinil inhalation solution to TreT is safe, well-tolerated, and accompanied by statistically significant improvements in key clinical assessments and patient-reported outcomes with comparable systemic exposure between the two formulations at evaluated doses (trial registration: clinicaltrials.gov identifier: NCT03950739).
Keywords: PAH‐SYMPACT; dry powder inhaler; pharmacokinetics; quality of life; treprostinil.
© 2022 United Therapeutics Corporation. United Therapeutics Corporation published by John Wiley & Sons Ltd on behalf of Pulmonary Vascular Research Institute.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.A.B. has received funding from Janssen, Bayer, and Actelion for research and honoraria from United Therapeutics Corporation, Bayer, Janssen, AstraZeneca, Intuitive, and Boehringer Ingelheim for lectures, presentations, or speakers' bureau. C.D.B. has no conflict of interest. C.D. is an employee of United Therapeutics Corporation. S.V.D. has no conflict of interest. M.S.E. reports ongoing industry‐sponsored research with Acceleron, Merck, and United Therapeutics Corporation. K.A.E. has received fees from United Therapeutics Corporation for serving on a speakers' bureau and advisory boards; from J&J Actelion for serving on advisory boards; and from Acceleron for consulting services; and institutional research funding from UT and J&J Actelion. M.R.F. has no conflict of interest. S.J. has received research funding from United Therapeutics Corporation and Bellerophon Therapeutics and honoraria from Actelion and Bayer for speakers programs. J.M. has received speaker fees from United Therapeutics Corporation. M.M. is an employee of United Therapeutics Corporation. J.M.J. has no conflict of interest. H.I.P. has received honoraria from Acceleron, United Therapeutics Corporation, and Actelion‐Janssen for serving on advisory boards. G.V.R. has no conflict of interest. R.R.J. has received fees from United Therapeutics Corporation, Bayer, and J&J Actelion for consulting, serving on a speakers' bureau, and research funding. S.S. has served as a consultant for United Therapeutics Corporation, Acceleron, Actelion, and Bayer Pharmaceuticals; as an advisor and speaker for United Therapeutics Corporation, Actelion, and Bayer; and as clinical trial site PI for United Therapeutics Corporation, Actelion, Merck, Liquidia Technologies, Altavant Sciences, and Gossamer Bio; and has received research grant support from ACCP CHEST and United Therapeutics Corporation. T.G.S. has served as an advisor for Bayer Pharmaceuticals and Liquidia Technologies and as clinical trial site PI for United Therapeutics Corporation, Actelion/J &J, Liquidia Technologies, Bayer Pharmaceuticals, and Regeneron Pharmaceuticals. S.M.S. has no conflict of interest. P.S. is an employee of United Therapeutics Corporation. L.A.S. has received consulting fees from Gossamer Bio and has served as clinical trial site PI for United Therapeutics Corporation, Gossamer Bio, INSMED, Actelion, Liquidia, Merck, and Altavant Sciences.
Figures

References
-
- Rubin LJ. Primary pulmonary hypertension. New Engl J Med. 1997;336:111–7. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

