Critical role for the lung endothelial nonmuscle myosin light-chain kinase isoform in the severity of inflammatory murine lung injury
- PMID: 35514774
- PMCID: PMC9063969
- DOI: 10.1002/pul2.12061
Critical role for the lung endothelial nonmuscle myosin light-chain kinase isoform in the severity of inflammatory murine lung injury
Abstract
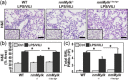
Global knockout of the nonmuscle isoform of myosin light-chain kinase (nmMLCK), a primary cellular regulator of cytoskeletal machinery, is strongly protective in preclinical murine models of inflammatory lung injury. The current study was designed to assess the specific contribution of endothelial cell (EC) nmMLCK to the severity of murine inflammatory lung injury produced by lipopolysaccharide (LPS) and mechanical ventilation ventilator-induced lung injury or ventilation (VILI). Responses to combined LPS/VILI exposure were assessed in: (i) wild-type (WT) C57BL/6J mice; (ii) transgenic mice with global deletion of nmMLCK (nmMylk -/-); (iii) transgenic nmMylk -/- mice with overexpression of nmMLCK restricted to the endothelium (nmMylk -/-/ec-tg+). Lung inflammation indices included lung histology, bronchoalveolar lavage (BAL) polymorphonuclear leukocytes (PMNs), lung protein biochemistry, tissue albumin levels, Evans blue dye (EBD) lung extravasation, and plasma cytokines (interleukin-6 [IL-6], keratinocyte chemoattractant [KC]/IL-8, IL-1bβ, extracellular nicotinamide phosphoribosyltransferase, tumor necrosis factor-α). Compared to WT C57BL/6J mice, the severity of LPS/VILI-induced lung injury was markedly reduced in mice with global nmMLCK deletion reflected by reductions in histologic inflammatory lung injury, BAL PMN counts, mitogen-activated protein kinase, and NF-kB pathway activation in lung homogenates, plasma cytokine levels, and parameters of lung permeability (increased BAL protein, tissue albumin levels, EBD lung extravasation). In contrast, mice with restricted overexpression of nmMLCK in EC (nmMylk -/-/ec-tg+) showed significant persistence of LPS/VILI-induced lung injury severity compared to WT mice. In conclusion, these studies strongly endorse the role of EC nmMLCK in driving the severity of preclinical inflammatory lung injury. Precise targeting of EC nmMLCK may represent an attractive therapeutic strategy to reduce lung inflammation and both lung and systemic vascular permeability.
Keywords: ARDS; MLCK.
© 2022 The Authors. Pulmonary Circulation published by Wiley Periodicals LLC on behalf of the Pulmonary Vascular Research Institute.
Conflict of interest statement
Joe G. N. Garcia is CEO and founder of Aqualung Therapeutics Corporation. The remaining authors declare no conflicts of interest.
Figures



References
-
- Garcia JG, Lazar V, Gilbert‐McClain LI, Gallagher PJ, Verin AD. Myosin light chain kinase in endothelium: molecular cloning and regulation. Am J Respir Cell Mol Biol. 1997;16:489–94. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

