Effect of calcination temperature, pH and catalyst loading on photodegradation efficiency of urea derived graphitic carbon nitride towards methylene blue dye solution
- PMID: 35514817
- PMCID: PMC9064223
- DOI: 10.1039/c9ra02201e
Effect of calcination temperature, pH and catalyst loading on photodegradation efficiency of urea derived graphitic carbon nitride towards methylene blue dye solution
Abstract
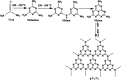
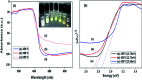
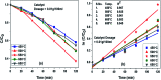
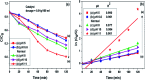
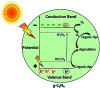
In this study, the photodegradation of methylene blue (MB) dye was performed using urea based graphitic carbon nitride (g-C3N4). Interestingly, it has been observed that the calcination temperature for the synthesis of g-C3N4 along with factors (pH and catalyst loading) influencing the photodegradation process, can make an impactful improvement in its photodegradation activity towards MB dye solution. The concept behind the comparatively improved photoactivity of g-C3N4 prepared at 550 °C was explored using various characterisation techniques like XRD, FTIR, SEM, BET and DRS. The FTIR and XRD patterns demonstrated that synthesis of g-C3N4 took place properly only when the calcination temperature was above 450 °C. The evolution of morphological and optical properties based on calcination temperature led to dramatically increased BET surface area and a decreased optical band gap value of g-C3N4 prepared at 550 °C. The effects of pH conditions and catalyst concentration on the MB dye degradation rate using optimally synthesised g-C3N4 are discussed. The value of the apparent rate constant was found to be 12 times more in the case of photodegradation of the MB dye using g-C3N4 prepared at 550 °C at optimum pH and catalyst loading conditions when compared with g-C3N4 prepared at 450 °C showing the lowest photoactivity potential. Further, high stability of the photocatalyst was observed for four cyclic runs of the photocatalytic reaction. Hence, g-C3N4 can be considered as a potential candidate for methylene blue photodegradation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Single-Step Synthesis of Graphitic Carbon Nitride Nanomaterials by Directly Calcining the Mixture of Urea and Thiourea: Application for Rhodamine B (RhB) Dye Degradation.Nanomaterials (Basel). 2023 Feb 17;13(4):762. doi: 10.3390/nano13040762. Nanomaterials (Basel). 2023. PMID: 36839130 Free PMC article.
-
Synthesis of polymeric 2D-graphitic carbon nitride (g-C3N4) nanosheets for sustainable photodegradation of organic pollutants.Heliyon. 2024 Jun 25;10(13):e33354. doi: 10.1016/j.heliyon.2024.e33354. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39040285 Free PMC article.
-
Efficient photocatalytic degradation of textile dye pollutants using thermally exfoliated graphitic carbon nitride (TE-g-C3N4).Sci Rep. 2024 Jan 27;14(1):2284. doi: 10.1038/s41598-024-52688-y. Sci Rep. 2024. PMID: 38280908 Free PMC article.
-
Graphitic carbon nitride based nanocomposites for the photocatalysis of organic contaminants under visible irradiation: Progress, limitations and future directions.Sci Total Environ. 2018 Aug 15;633:546-559. doi: 10.1016/j.scitotenv.2018.03.206. Epub 2018 Mar 28. Sci Total Environ. 2018. PMID: 29579666 Review.
-
g-C3N4 Based Photocatalyst for the Efficient Photodegradation of Toxic Methyl Orange Dye: Recent Modifications and Future Perspectives.Molecules. 2023 Apr 4;28(7):3199. doi: 10.3390/molecules28073199. Molecules. 2023. PMID: 37049963 Free PMC article. Review.
Cited by
-
Photocatalytic degradation of methylene blue under natural sunlight using iron titanate nanoparticles prepared by a modified sol-gel method.R Soc Open Sci. 2020 Sep 2;7(9):200708. doi: 10.1098/rsos.200708. eCollection 2020 Sep. R Soc Open Sci. 2020. PMID: 33047033 Free PMC article.
-
A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)-Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing.Nanomaterials (Basel). 2022 Jan 17;12(2):294. doi: 10.3390/nano12020294. Nanomaterials (Basel). 2022. PMID: 35055311 Free PMC article. Review.
-
High-Efficiency Ag-Modified ZnO/g-C3N4 Photocatalyst with 1D-0D-2D Morphology for Methylene Blue Degradation.Molecules. 2024 May 7;29(10):2182. doi: 10.3390/molecules29102182. Molecules. 2024. PMID: 38792044 Free PMC article.
-
An Efficient p-n Heterojunction Copper Tin Sulfide/g-C3N4 Nanocomposite for Methyl Orange Photodegradation.ACS Omega. 2024 Jun 17;9(26):28463-28475. doi: 10.1021/acsomega.4c02414. eCollection 2024 Jul 2. ACS Omega. 2024. PMID: 38973891 Free PMC article.
-
Enhanced visible light photocatalytic activity of Fe2O3 modified TiO2 prepared by atomic layer deposition.Sci Rep. 2020 Aug 10;10(1):13437. doi: 10.1038/s41598-020-70352-z. Sci Rep. 2020. PMID: 32778781 Free PMC article.
References
-
- Andreozzi R. Catal. Today. 1999;53:51–59. doi: 10.1016/S0920-5861(99)00102-9. - DOI
-
- Quiroz M. A., Bandala E. R. and Martinez-Huitle C. A., Advanced Oxidation Processes (AOPs) for Removal of Pesticides from Aqueous Media, Pesticides - Formulations, Effects, Fate, ed. M. Stoytcheva, 2011
-
- Binas V. Venieri D. Kotzias D. Kiriakidis G. J. Materiomics. 2017;3:3–16. doi: 10.1016/j.jmat.2016.11.002. - DOI
-
- Ibhadon A. Fitzpatrick P. Catalysts. 2013;3:189–218. doi: 10.3390/catal3010189. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

