A magnetically separable plate-like cadmium titanate-copper ferrite nanocomposite with enhanced visible-light photocatalytic degradation performance for organic contaminants
- PMID: 35514850
- PMCID: PMC9064311
- DOI: 10.1039/c9ra01968e
A magnetically separable plate-like cadmium titanate-copper ferrite nanocomposite with enhanced visible-light photocatalytic degradation performance for organic contaminants
Abstract
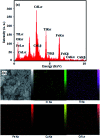
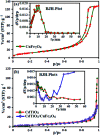
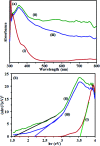
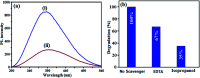
A novel magnetic cadmium titanate-copper ferrite (CdTiO3/CuFe2O4) nanocomposite, in which spherical CuFe2O4 nanoparticles were loaded onto the surface of CdTiO3 nanoplates, was successfully synthesized via a sol-gel hydrothermal route at 180 °C. The structure, morphology, magnetic and optical properties of the as-prepared nanocomposite were respectively characterized by Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), energy dispersive X-ray (EDX) spectroscopy, transmission electron microscopy (TEM), Brunauer-Emmett-Teller (BET) surface area analysis, UV-visible diffuse reflectance spectroscopy (DRS), vibrating sample magnetometry (VSM) and photoluminescence (PL) spectroscopy. The photocatalytic activity of this novel CdTiO3-based magnetic nanocomposite was investigated for the degradation of organic dye pollutants such as methylene blue (MB), rhodamine B (RhB), and methyl orange (MO) in the presence of H2O2 under visible light irradiation. The results showed that the photocatalyst completely degraded three dyes within 90-100 min. Compared with pure CdTiO3 and CuFe2O4, the heterogeneous CdTiO3/CuFe2O4 nanocomposite exhibited significantly enhanced photocatalytic efficiency. On the basis of the results of the OH trapping and photoluminescence (PL) experiments, the enhanced photocatalytic performance was mainly ascribed to the efficient separation of photo-induced electron-hole pairs and the formation of highly active hydroxyl radicals (OH) species in the CdTiO3/CuFe2O4 photocatalytic oxidation system. The PL measurements of the CdTiO3/CuFe2O4 nanocomposite also indicated an enhanced separation of photo-induced electron-hole pairs. Moreover, the nanocomposite could be easily separated and recycled from contaminant solution using a magnet without a decrease in their photocatalytic activity due to their good magnetic separation performance and excellent chemical stability. Based on these findings, CdTiO3/CuFe2O4 nanocomposite could be a promising visible-light-driven magnetic photocatalyst for converting solar energy to chemical energy for environmental remediation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures










Similar articles
-
A novel n-type CdS nanorods/p-type LaFeO3 heterojunction nanocomposite with enhanced visible-light photocatalytic performance.RSC Adv. 2019 Aug 7;9(42):24489-24504. doi: 10.1039/c9ra04265b. eCollection 2019 Aug 2. RSC Adv. 2019. PMID: 35527888 Free PMC article.
-
Synthesis of magnetic graphene-like carbon nitride-cobalt ferrite (g-C3N4/CoFe2O4) nanocomposite for sonocatalytic remediation of toxic organic dyes.RSC Adv. 2023 Apr 6;13(16):10940-10955. doi: 10.1039/d3ra00057e. eCollection 2023 Apr 3. RSC Adv. 2023. PMID: 37033431 Free PMC article.
-
Coupling ZnO with CuO for efficient organic pollutant removal.Environ Sci Pollut Res Int. 2023 Jun;30(28):71984-72008. doi: 10.1007/s11356-022-24139-6. Epub 2022 Nov 22. Environ Sci Pollut Res Int. 2023. PMID: 36414902
-
Efficient photocatalytic degradation of organic pollutants by magnetically recoverable nitrogen-doped TiO2 nanocomposite photocatalysts under visible light irradiation.Environ Sci Pollut Res Int. 2015 Dec;22(23):18859-73. doi: 10.1007/s11356-015-5032-3. Epub 2015 Jul 24. Environ Sci Pollut Res Int. 2015. PMID: 26206125
-
A facile synthesis process of GCN/ZnO-Cu nanocomposite and the evaluation of the performance for the photocatalytic degradation of organic pollutants and the disinfection of wastewater under visible light.Chemosphere. 2023 Dec;344:140287. doi: 10.1016/j.chemosphere.2023.140287. Epub 2023 Oct 10. Chemosphere. 2023. PMID: 37820879 Review.
Cited by
-
CdTiO3-NPs incorporated TiO2 nanostructure photocatalyst for scavenger-free water splitting under visible radiation.PLoS One. 2022 Oct 18;17(10):e0276097. doi: 10.1371/journal.pone.0276097. eCollection 2022. PLoS One. 2022. PMID: 36256606 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

