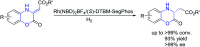Efficient access to chiral dihydrobenzoxazinones via Rh-catalyzed hydrogenation
- PMID: 35514854
- PMCID: PMC9064260
- DOI: 10.1039/c9ra02694k
Efficient access to chiral dihydrobenzoxazinones via Rh-catalyzed hydrogenation
Abstract
Rh/(S)-DTBM-SegPhos-catalyzed asymmetric hydrogenation of prochiral (Z)-2-(2-oxo-2H-benzo[b][1,4]oxazin-3(4H)-ylidene)acetate esters was successfully developed. A series of chiral dihydrobenzoxazinones were prepared through this efficient methodology with good to excellent results (up to >99% conversion, 93% yield and >99% ee), which are important motifs in the biologically active molecules.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Rh-Catalyzed Asymmetric Hydrogenation of α,β- and β,β-Disubstituted Unsaturated Boronate Esters.Chemistry. 2020 May 12;26(27):5961-5964. doi: 10.1002/chem.202000703. Epub 2020 Apr 28. Chemistry. 2020. PMID: 32048767
-
Enantioselective Access to Chiral 2-Substituted 2,3-Dihydrobenzo[1,4]dioxane Derivatives through Rh-Catalyzed Asymmetric Hydrogenation.Org Lett. 2018 Jul 20;20(14):4173-4177. doi: 10.1021/acs.orglett.8b01469. Epub 2018 Jul 3. Org Lett. 2018. PMID: 29968478
-
Efficient synthesis of chiral 2,3-dihydro-benzo[b]thiophene 1,1-dioxides via Rh-catalyzed hydrogenation.Chem Sci. 2019 Jan 15;10(8):2507-2512. doi: 10.1039/c8sc05397a. eCollection 2019 Feb 28. Chem Sci. 2019. PMID: 30881681 Free PMC article.
-
Rh-Catalyzed Asymmetric Hydrogenation of α-Substituted Vinyl Sulfones: An Efficient Approach to Chiral Sulfones.Org Lett. 2017 Mar 3;19(5):1024-1027. doi: 10.1021/acs.orglett.6b03845. Epub 2017 Feb 23. Org Lett. 2017. PMID: 28229596
-
Diastereo- and enantioselective anti-selective hydrogenation of α-amino-β-keto ester hydrochlorides and related compounds using transition-metal-chiral-bisphosphine catalysts.Chem Rec. 2014 Apr;14(2):235-50. doi: 10.1002/tcr.201300032. Epub 2014 Feb 18. Chem Rec. 2014. PMID: 24550034 Review.
References
-
- Smil D. V. Manku S. Chantigny Y. A. Leit S. Wahhab A. Deziel R. Yan T. P. Fournel M. Maroun C. Li Z. Lemieux A.-M. Nicolescu A. Rahil J. Lefebvre S. Panetta A. Besterman J. M. Lefebvre S. Panetta A. Besterman J. M. Déziel R. Bioorg. Med. Chem. Lett. 2009;19:688–692. doi: 10.1016/j.bmcl.2008.12.045. - DOI - PubMed
- Mahaney P. E. Webb M. B. Ye F. Sabatucci J. P. Steffan R. J. Chadwick C. C. Harnish D. C. Trybulski E. J. Bioorg. Med. Chem. 2006;14:3455–3466. doi: 10.1016/j.bmc.2006.01.001. - DOI - PubMed
- Su D.-S. Markowitz M. K. DiPardo R. M. Murphy K. L. Harrell C. M. O'Malley S. S. Ransom R. W. Chang R. S. L. Ha S. Hess F. J. Pettibone D. J. Mason G. S. Boyce S. Freidinger R. M. Bock M. G. J. Am. Chem. Soc. 2003;125:7516–7517. doi: 10.1021/ja0353457. - DOI - PubMed
- Grant F., Bartulis S., Brogley L., Dappan M. S., Kasar R., Khan A., Neitzel M., Pleiss M. A., Thorsett E. D., Tucker J., Ye M. and Hawkinson J., PCT Int. Appl., WO 2003093245 A1, 2003
- Jones Z., Groneberg R., Drew M. and Eary C. T., US Pat., 20050282812 A1, 2005
- Su D.-S. and Bock M. G., U.S. Pat. Appl. Publ. US 20050020591 A1, 2005
- Ilaš J. Anderluh P. Š. Dolenc M. S. Kikelj D. Tetrahedron. 2005;61:7325–7348. doi: 10.1016/j.tet.2005.05.037. - DOI
- Abraham C. J. Paull D. H. Scerba M. T. Grebinski J. W. Lectka T. J. Am. Chem. Soc. 2006;128:13370–13371. doi: 10.1021/ja065754d. - DOI - PubMed
- Rueping M. Stoeckel M. Sugiono E. Theissmann T. Tetrahedron. 2010;66:6565–6568. doi: 10.1016/j.tet.2010.04.091. - DOI
-
- Hayward C. M. and Scully D. A., US Pat., US 6207664 B1, 2001
- Tanaka T. Mizota I. Umezu K. Ito A. Shimizu M. Heterocycles. 2017;95:830–843. doi: 10.3987/COM-16-S(S)27. - DOI
-
- Chen J. J. Qian W. Biswas K. Viswanadhan V. N. Askew B. C. Hitchcock S. Hungate R. W. Arik L. Johnson E. Bioorg. Med. Chem. Lett. 2008;18:4477–4481. doi: 10.1016/j.bmcl.2008.07.055. - DOI - PubMed
- Chen M.-W. Deng Z. Yang Q. Huang J. Peng Y. Org. Chem. Front. 2019;6:746–750. doi: 10.1039/C8QO01361F. - DOI
-
- Michael S. S.-S., WO2012151440, 2012
- Zhang X. Xu B. Xu M.-H. Org. Chem. Front. 2016;3:944–948. doi: 10.1039/C6QO00191B. - DOI
LinkOut - more resources
Full Text Sources