Two decades of recent advances of Ugi reactions: synthetic and pharmaceutical applications
- PMID: 35514898
- PMCID: PMC9058431
- DOI: 10.1039/d0ra07501a
Two decades of recent advances of Ugi reactions: synthetic and pharmaceutical applications
Abstract
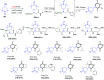
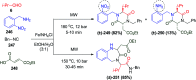
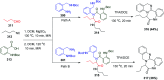
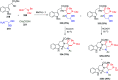
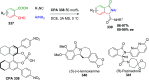
Multicomponent reactions (MCRs) are powerful synthetic tools in which more than two starting materials couple with each other to form multi-functionalized compounds in a one-pot process, the so-called "tandem", "domino" or "cascade" reaction, or utilizing an additional step without changing the solvent, the so-called a sequential-addition procedure, to limit the number of synthetic steps, while increasing the complexity and the molecular diversity, which are highly step-economical reactions. The Ugi reaction, one of the most common multicomponent reactions, has recently fascinated chemists with the high diversity brought by its four- or three-component-based isonitrile. The Ugi reaction has been introduced in organic synthesis as a novel, efficient and useful tool for the preparation of libraries of multifunctional peptides, natural products, and heterocyclic compounds with stereochemistry control. In this review, we highlight the recent advances of the Ugi reaction in the last two decades from 2000-2019, mainly in the synthesis of linear or cyclic peptides, heterocyclic compounds with versatile ring sizes, and natural products, as well as the enantioselective Ugi reactions. Meanwhile, the applications of these compounds in pharmaceutical trials are also discussed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures



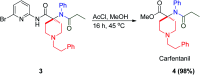
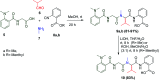


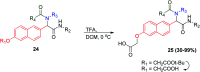

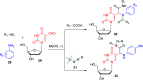






























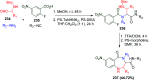

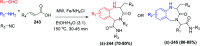












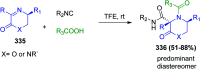
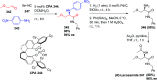
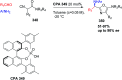
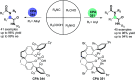
Similar articles
-
Cyclic Imines in Ugi and Ugi-Type Reactions.ACS Comb Sci. 2020 Aug 10;22(8):361-400. doi: 10.1021/acscombsci.0c00046. Epub 2020 Jul 14. ACS Comb Sci. 2020. PMID: 32574488 Review.
-
Post-Ugi Cyclization for the Construction of Diverse Heterocyclic Compounds: Recent Updates.Front Chem. 2018 Nov 20;6:557. doi: 10.3389/fchem.2018.00557. eCollection 2018. Front Chem. 2018. PMID: 30525022 Free PMC article. Review.
-
Brønsted-acid-catalyzed asymmetric multicomponent reactions for the facile synthesis of highly enantioenriched structurally diverse nitrogenous heterocycles.Acc Chem Res. 2011 Nov 15;44(11):1156-71. doi: 10.1021/ar2000343. Epub 2011 Jul 29. Acc Chem Res. 2011. PMID: 21800828
-
Isocyanide-Based Multicomponent Reactions in Water: Advanced Green Tools for the Synthesis of Heterocyclic Compounds.Top Curr Chem (Cham). 2022 Sep 22;380(6):50. doi: 10.1007/s41061-022-00403-8. Top Curr Chem (Cham). 2022. PMID: 36136281 Review.
-
Recent advances in the development of polycyclic skeletons via Ugi reaction cascades.Mol Divers. 2018 May;22(2):503-516. doi: 10.1007/s11030-017-9811-2. Epub 2018 Jan 16. Mol Divers. 2018. PMID: 29340996 Review.
Cited by
-
Design, synthesis, and evaluation of 1,4-benzothiazine-3-one containing bisamide derivatives as dual inhibitors of Staphylococcus aureus with plausible application in a urinary catheter.Front Chem. 2024 Jun 26;12:1420593. doi: 10.3389/fchem.2024.1420593. eCollection 2024. Front Chem. 2024. PMID: 38988728 Free PMC article.
-
Versatile, Modular, and General Strategy for the Synthesis of α-Amino Carbonyls.J Am Chem Soc. 2024 Sep 4;146(35):24699-24707. doi: 10.1021/jacs.4c09434. Epub 2024 Aug 24. J Am Chem Soc. 2024. PMID: 39180740 Free PMC article.
-
Excellent Catalytic Performances of a Au/C-CuO Binary System in the Selective Oxidation of Benzylamines to Imines under Atmospheric Oxygen.ACS Omega. 2021 Dec 6;6(50):34339-34346. doi: 10.1021/acsomega.1c04046. eCollection 2021 Dec 21. ACS Omega. 2021. PMID: 34963919 Free PMC article.
-
Multicomponent Reaction-Assisted Drug Discovery: A Time- and Cost-Effective Green Approach Speeding Up Identification and Optimization of Anticancer Drugs.Int J Mol Sci. 2023 Apr 1;24(7):6581. doi: 10.3390/ijms24076581. Int J Mol Sci. 2023. PMID: 37047554 Free PMC article. Review.
-
Multicomponent reactions driving the discovery and optimization of agents targeting central nervous system pathologies.Beilstein J Org Chem. 2024 Dec 3;20:3151-3173. doi: 10.3762/bjoc.20.261. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 39669443 Free PMC article. Review.
References
-
- Janežič D. Hodošček M. Ugi I. Internet Electron. J. Mol. Des. 2002;1:293–299.
-
- Kumar S. Mukesh K. Harjai K. Singh V. Tetrahedron Lett. 2019;60:8–12. doi: 10.1016/j.tetlet.2018.11.030. - DOI
-
- Passerini M. Simone L. Gazz. Chim. Ital. 1921;51:126–129.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

