Approach to nigericin derivatives and their therapeutic potential
- PMID: 35514935
- PMCID: PMC9058090
- DOI: 10.1039/d0ra05137c
Approach to nigericin derivatives and their therapeutic potential
Abstract
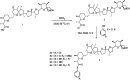
A new nigericin analogue that has been chemically modified was synthesized through a fluorination process from the parent nigericin, produced from a novel Streptomyces strain DASNCL-29. Fermentation strategies were designed for the optimised production of nigericin molecule and subjected for purification and structural analysis. The fermentation process resulted in the highest yield of nigericin (33% (w/w)). Initially, nigericin produced from the strain DASNCL-29 demonstrated polymorphism in its crystal structure, i.e., monoclinic and orthorhombic crystal lattices when crystallised with methanol and hexane, respectively. Furthermore, nigericin produced has been subjected to chemical modification by fluorination to enhance its efficacy. Two fluorinated analogues revealed that they possess a very potent antibacterial activity against Gram positive and Gram negative bacteria. To date, the nigericin molecule has not been reported for any reaction against Gram-negative bacteria, which are increasingly becoming resistant to antibiotics. For the first time, fluorinated analogues of nigericin have shown promising activity. In vitro cytotoxicity analysis of fluorinated analogues demonstrated tenfold lesser toxicity than the parent nigericin. This is the first type of study where the fluorinated analogues of nigericin showed very encouraging activity against Gram-negative organisms; moreover, they can be used as a candidate for treating many serious infections.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Song M. Liu Y. Huang X. Ding S. Wang Y. Shen J. et al., A broad-spectrum antibiotic adjuvant reverses multidrug-resistant Gram-negative pathogens. Nat. Microbiol. 2020:1–11. - PubMed
-
- World Health Organization, Antibiotic resistance threats in the United States, 2019
-
- Boucher H. W. Talbot G. H. Benjamin D. K. Bradley J. Guidos R. J. Jones R. N. et al., 10 × ’20 progress – development of new drugs active against gram-negative bacilli: an update from the infectious diseases society of America. Clin. Infect. Dis. 2013;56(12):1685–1694. doi: 10.1093/cid/cit152. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

