Synthesis of novel naphthalene-heterocycle hybrids with potent antitumor, anti-inflammatory and antituberculosis activities
- PMID: 35514936
- PMCID: PMC9058152
- DOI: 10.1039/d0ra08526j
Synthesis of novel naphthalene-heterocycle hybrids with potent antitumor, anti-inflammatory and antituberculosis activities
Abstract
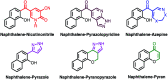
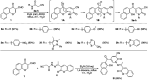
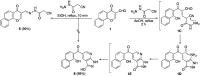
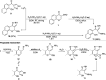
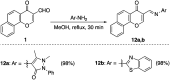
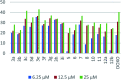
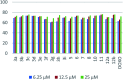
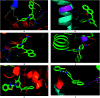
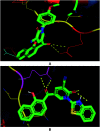
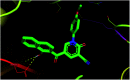
Multitarget-directed drugs (hybrid drugs) constitute an efficient avenue for the treatment of multifactorial diseases. In this work, novel naphthalene hybrids with different heterocyclic scaffolds such as nicotinonitrile, pyran, pyranopyrazole, pyrazole, pyrazolopyridine, and azepine were efficiently synthesized via tandem reactions of 3-formyl-4H-benzo[h]chromen-4-one 1 with different nucleophilic reagents. Analysis of these hybrids using PASS online software indicated different predicted biological activities such as anticancer, antimicrobial, antiviral, antiprotozoal, anti-inflammatory, etc. By focusing on antitumor, anti-inflammatory, and antituberculosis activities, many compounds revealed remarkable activities. While 3c, 3e, and 3h were more potent than doxorubicin in the case of HepG-2 cell lines, 3a-e, 3i, 6, 8, 10, 11, and 12b were more potent in the case of MCF-7. Moreover, compounds 3c, 3h, 8, 10, 3d, and 12b manifested superior activity and COX-2 selectivity to the reference anti-inflammatory Celecoxib. Regarding antituberculosis activity, 3c, 3d, and 3i were found to be the most promising with MIC less than 1 μg mL-1. The molecular docking studies showed strong polar and hydrophobic interactions with the novel naphthalene-heterocycle hybrids that were compatible with experimental evaluations to a great extent.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

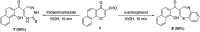
Similar articles
-
Synthesis, Molecular Docking Study and in vitro Anticancer Activity of Tetrazole Linked Benzochromene Derivatives.Anticancer Agents Med Chem. 2017;17(3):464-470. doi: 10.2174/1871520616666160627090249. Anticancer Agents Med Chem. 2017. PMID: 27357544
-
Microwave-assisted synthesis, computational studies and antibacterial/ anti-inflammatory activities of compounds based on coumarin-pyrazole hybrid.R Soc Open Sci. 2018 May 2;5(5):172435. doi: 10.1098/rsos.172435. eCollection 2018 May. R Soc Open Sci. 2018. PMID: 29892430 Free PMC article.
-
New 1,2,4-triazole/pyrazole hybrids linked to oxime moiety as nitric oxide donor celecoxib analogs: Synthesis, cyclooxygenase inhibition anti-inflammatory, ulcerogenicity, anti-proliferative activities, apoptosis, molecular modeling and nitric oxide release studies.Bioorg Chem. 2020 May;98:103752. doi: 10.1016/j.bioorg.2020.103752. Epub 2020 Mar 12. Bioorg Chem. 2020. PMID: 32197148
-
A Review of the Recent Development in the Synthesis and Biological Evaluations of Pyrazole Derivatives.Biomedicines. 2022 May 12;10(5):1124. doi: 10.3390/biomedicines10051124. Biomedicines. 2022. PMID: 35625859 Free PMC article. Review.
-
Synthesis of Antimicrobial Benzimidazole-Pyrazole Compounds and Their Biological Activities.Antibiotics (Basel). 2021 Aug 19;10(8):1002. doi: 10.3390/antibiotics10081002. Antibiotics (Basel). 2021. PMID: 34439052 Free PMC article. Review.
Cited by
-
Acute Oral, Subacute, and Developmental Toxicity Profiling of Naphthalene 2-Yl, 2-Chloro, 5-Nitrobenzoate: Assessment Based on Stress Response, Toxicity, and Adverse Outcome Pathways.Front Pharmacol. 2022 Jan 20;12:810704. doi: 10.3389/fphar.2021.810704. eCollection 2021. Front Pharmacol. 2022. PMID: 35126145 Free PMC article.
-
Naphthyl bearing 1,3,4-thiadiazoleacetamides targeting the parasitic folate pathway as anti-infectious agents: in silico, synthesis, and biological approach.RSC Med Chem. 2023 Nov 21;14(12):2768-2781. doi: 10.1039/d3md00423f. eCollection 2023 Dec 13. RSC Med Chem. 2023. PMID: 38107179 Free PMC article.
-
Recent advances in pyrazolo[3,4-b]pyridine chemistry: synthesis techniques and biological activity.Mol Divers. 2025 Jun 25. doi: 10.1007/s11030-025-11231-5. Online ahead of print. Mol Divers. 2025. PMID: 40560489 Review.
-
Rigid 3D-spiro chromanone as a crux for efficient antimicrobial agents: synthesis, biological and computational evaluation.RSC Adv. 2021 Jun 16;11(35):21301-21314. doi: 10.1039/d1ra03497a. eCollection 2021 Jun 15. RSC Adv. 2021. PMID: 35478839 Free PMC article.
-
Recent advances in the synthesis of new benzothiazole based anti-tubercular compounds.RSC Adv. 2023 Jul 21;13(32):21890-21925. doi: 10.1039/d3ra03862a. eCollection 2023 Jul 19. RSC Adv. 2023. PMID: 37483662 Free PMC article. Review.
References
-
- El-Desoky S. I. Keshk E. M. El-Sawi A. A. Abozeid M. A. Abouzeid L. A. Abdel-Rahman A. H. Saudi Pharm. J. 2018;26:852–859. doi: 10.1016/j.jsps.2018.03.013. - DOI - PMC - PubMed
- Abozeid M. A. El-Kholany M. R. Abdel-Rahman A. H. El-Desoky S. I. Int. J. Mod. Org. Chem. 2018;5:1–11.
- Abozeid M. A. El-Kholany M. R. Abouzeid L. A. Abdel-Rahman A. H. El-Desoky S. I. J. Heterocycl. Chem. 2019;56:2922–2933. doi: 10.1002/jhet.3686. - DOI
- El-Desoky S. I. El-Sawi A. A. Abozeid M. A. Abdelmoteleb M. Shaaban M. Keshk E. M. Abdel-Rahman A. H. Med. Chem. Res. 2019;28:1601–1617. doi: 10.1007/s00044-019-02397-3. - DOI
- Abozeid M. A. El-Sawi A. A. Elmorsy M. R. Abdelmoteleb M. Abdel-Rahman A. H. El-Desoky S. I. RSC Adv. 2019;9:27996–28005. doi: 10.1039/C9RA05154F. - DOI - PMC - PubMed
-
- Valente S. Trisciuoglio D. De Luca T. Nebbioso A. Labella D. Lenoci A. Bigogno C. Dondio G. Miceli M. Brosch G. J. Med. Chem. 2014;57:6259–6265. doi: 10.1021/jm500303u. - DOI - PubMed
- Abate C. Niso M. Lacivita E. Mosier P. D. Toscano A. Perrone R. J. Med. Chem. 2011;54:1022–1032. doi: 10.1021/jm1013133. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

