Emulation of a Target Trial From Observational Data to Compare Effectiveness of Casirivimab/Imdevimab and Bamlanivimab/Etesevimab for Early Treatment of Non-Hospitalized Patients With COVID-19
- PMID: 35514955
- PMCID: PMC9066636
- DOI: 10.3389/fimmu.2022.868020
Emulation of a Target Trial From Observational Data to Compare Effectiveness of Casirivimab/Imdevimab and Bamlanivimab/Etesevimab for Early Treatment of Non-Hospitalized Patients With COVID-19
Abstract
Objectives: Comparative analysis between different monoclonal antibodies (mAbs) against SARS-CoV-2 are lacking. We present an emulation trial from observational data to compare effectiveness of Bamlanivimab/Etesevimab (BAM/ETE) and Casirivimab/Imdevimab (CAS/IMD) in outpatients with early mild-to-moderate COVID-19 in a real-world scenario of variants of concern (VoCs) from Alpha to Delta.
Methods: Allocation to treatment was subject to mAbs availability, and the measured factors were not used to determine which combination to use. Patients were followed through day 30. Viral load was measured by cycle threshold (CT) on D1 (baseline) and D7.Primary outcome was time to COVID-19-related hospitalization or death from any cause over days 0-30. Weighted pooled logistic regression and marginal structural Cox model by inverse probability weights were used to compare BAM/ETE vs. CAS/IMD. ANCOVA was used to compare mean D7 CT values by intervention. Models were adjusted for calendar month, MASS score and VoCs. We evaluated effect measure modification by VoCs, vaccination, D1 CT levels and enrolment period.
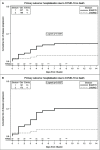
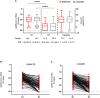
Results: COVID19-related hospitalization or death from any cause occurred in 15 of 237 patients in the BAM/ETE group (6.3%) and in 4 of 196 patients in the CAS/IMD group (2.0%) (relative risk reduction [1 minus the relative risk] 72%; p=0.024). Subset analysis carried no evidence that the effect of the intervention was different across stratification factors. There was no evidence in viral load reduction from baseline through day 7 across the two groups (+0.17, 95% -1.41;+1.74, p=0.83). Among patients who experienced primary outcome, none showed a negative RT-PCR test in nasopharyngeal swab (p=0.009) and 82.4% showed still high viral load (p<0.001) on D7.
Conclusions: In a pre-Omicron epidemiologic scenario, CAS/IMD reduced risk of clinical progression of COVID-19 compared to BAM/ETE. This effect was not associated with a concomitant difference in virological response.
Keywords: COVID-19; SARS-COV-2; bamlanivimab/etesevimab; casirivimab/imdevimab; early treatment for COVID-19; monoclonal antibodies.
Copyright © 2022 Mazzotta, Cozzi-Lepri, Colavita, Lanini, Rosati, Lalle, Mastrorosa, Cimaglia, Vergori, Bevilacqua, Lapa, Mariano, Bettini, Agrati, Piselli, Girardi, Castilletti, Garbuglia, Vaia, Nicastri and Antinori.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- COVID-19 Treatment Guidelines Panel . Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at: https://www.covid19treatmentguidelines.nih.gov/ (Accessed 28/12//2021). - PubMed
-
- Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. . Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA (2021) 325(7):632–44. doi: 10.1001/jama.2021.0202 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

