Characterization of Pannexin1, Connexin32, and Connexin43 in Spotted Sea Bass (Lateolabrax maculatus): They Are Important Neuro-Related Immune Response Genes Involved in Inflammation-Induced ATP Release
- PMID: 35514966
- PMCID: PMC9062032
- DOI: 10.3389/fimmu.2022.870679
Characterization of Pannexin1, Connexin32, and Connexin43 in Spotted Sea Bass (Lateolabrax maculatus): They Are Important Neuro-Related Immune Response Genes Involved in Inflammation-Induced ATP Release
Erratum in
-
Corrigendum: Characterization of pannexin1, connexin32, and connexin43 in spotted sea bass (Lateolabrax maculatus): they are important neuro-related immune response genes involved in inflammation-induced ATP release.Front Immunol. 2024 Apr 8;15:1377603. doi: 10.3389/fimmu.2024.1377603. eCollection 2024. Front Immunol. 2024. PMID: 38650923 Free PMC article.
Abstract
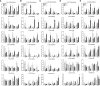
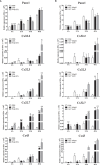
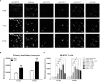
Many immunological diseases can be treated by regulating neurobehavior, in which extracellular ATP is a vital member of endogenous danger-associated molecular pattern signaling molecule that plays a crucial part in innate neuro-related immunity. It is actively released through pannexin (Panx) and connexin (Cx) hemichannels from activated or stressed cells during inflammation, injury, or apoptosis. In addition to participating in ATP release, Panxs and Cxs also have crucial immune functions. In this study, pannexin1, three connexin32 isoforms and connexin43 were identified and characterized in spotted sea bass (Lateolabrax maculatus), which were named LmPanx1, LmCx32.2, LmCx32.3, LmCx32.7, and LmCx43. Their similar topological structures were discovered by sequence analysis: a relatively unconserved C-terminal region and four highly conserved transmembrane (TM) domains, and so on. Each extracellular (ECL) region of Panx1 has two conserved cysteine residues. Unlike Panx1, each ECL region of Cx32 and Cx43 contains three conserved cysteine residues, forming two conserved motifs: CX6CX3C motif in ECL1 and CX4CX5C motif in ECL2. Furthermore, Panx1 and Cx43 share similar genomic organization and synteny with their counterparts in selected vertebrates. Cx32 and CX43 were located in the same locus in fish, but diverged into two loci from amphibian. Moreover, despite varying expression levels, the identified genes were constitutively expressed in all examined tissues. All genes were upregulated by PAMP [lipopolysaccharide and poly(I:C)] stimulation or bacterial infection in vivo and in vitro, but they were downregulated in the brain at 6 or 12 h after stimulation. Especially, the three LmCx32 isoforms and LmCx43 were upregulated by ATP stimulation in primary head kidney leukocytes; however, downregulation of LmCx32.3 and LmCx43 expression were noted at 12 h. Conversely, ATP treatment inhibited the expression of LmPanx1. Importantly, we showed that the spotted sea bass Panx1, Cx43, and Cx32 were localized on the cellular membrane and involved in inflammation-induced ATP release. Taken together, our results demonstrated that Panx1, Cx32, and Cx43 are important neuro-related immune response genes involved in inflammation-induced ATP release.
Keywords: ATP release; Lateolabrax maculatus; connexin32; connexin43; innate immunity; pannexin1.
Copyright © 2022 Sun, Xu, Chen, Liu, Wu and Gao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Activated P2X receptors can up-regulate the expressions of inflammation-related genes via NF-κB pathway in spotted sea bass (Lateolabrax maculatus).Front Immunol. 2023 May 4;14:1181067. doi: 10.3389/fimmu.2023.1181067. eCollection 2023. Front Immunol. 2023. PMID: 37215129 Free PMC article.
-
Characterization of three connexin32 genes and their role in inflammation-induced ATP release in the Japanese flounder Paralichthys olivaceus.Fish Shellfish Immunol. 2020 Nov;106:181-189. doi: 10.1016/j.fsi.2020.07.066. Epub 2020 Aug 6. Fish Shellfish Immunol. 2020. PMID: 32768708
-
Characterisation of P2X3 receptors in spotted sea bass (Lateolabrax maculatus): P2X3a and P2X3b mediate pro-inflammatory innate immune response.Fish Shellfish Immunol. 2025 Oct;165:110496. doi: 10.1016/j.fsi.2025.110496. Epub 2025 Jun 14. Fish Shellfish Immunol. 2025. PMID: 40523573
-
The role of pannexin1 in the induction and resolution of inflammation.FEBS Lett. 2014 Apr 17;588(8):1416-22. doi: 10.1016/j.febslet.2014.03.009. Epub 2014 Mar 15. FEBS Lett. 2014. PMID: 24642372 Free PMC article. Review.
-
Connexin 43 and Pannexin 1 hemichannels as endogenous regulators of innate immunity in sepsis.Front Immunol. 2024 Dec 23;15:1523306. doi: 10.3389/fimmu.2024.1523306. eCollection 2024. Front Immunol. 2024. PMID: 39763679 Free PMC article. Review.
Cited by
-
Activated P2X receptors can up-regulate the expressions of inflammation-related genes via NF-κB pathway in spotted sea bass (Lateolabrax maculatus).Front Immunol. 2023 May 4;14:1181067. doi: 10.3389/fimmu.2023.1181067. eCollection 2023. Front Immunol. 2023. PMID: 37215129 Free PMC article.
References
-
- Chen P, Wang Q, Wan X, Yang M, Liu C, Xu C, et al. . Wireless Electrical Stimulation of the Vagus Nerves by Ultrasound-Responsive Programmable Hydrogel Nanogenerators for Anti-Inflammatory Therapy in Sepsis. Nano Energy (2021) 89:106327. doi: 10.1016/j.nanoen.2021.106327 - DOI
-
- Chen P, Wu P, Wan X, Wang Q, Xu C, Yang M, et al. . Ultrasound-Driven Electrical Stimulation of Peripheral Nerves Based on Implantable Piezoelectric Thin Film Nanogenerators. Nano Energy (2021) 86:106123. doi: 10.1016/j.nanoen.2021.106123 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

