Ablation of BATF Alleviates Transplant Rejection via Abrogating the Effector Differentiation and Memory Responses of CD8+ T Cells
- PMID: 35514970
- PMCID: PMC9062028
- DOI: 10.3389/fimmu.2022.882721
Ablation of BATF Alleviates Transplant Rejection via Abrogating the Effector Differentiation and Memory Responses of CD8+ T Cells
Abstract
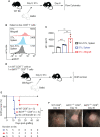
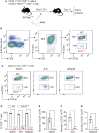
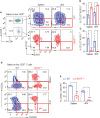
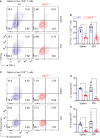
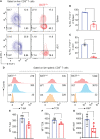
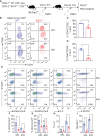
Allogeneic CD8+ T cells are prominently involved in allograft rejection, but how their effector differentiation and function are regulated at a transcriptional level is not fully understood. Herein, we identified the basic leucine zipper ATF-like transcription factor (BATF) as a key transcription factor that drives the effector program of allogeneic CD8+ T cells. We found that BATF is highly expressed in graft-infiltrating CD8+ T cells, and its ablation in CD8+ T cells significantly prolonged skin allograft survival in a fully MHC-mismatched transplantation model. To investigate how BATF dictates allogeneic CD8+ T cell response, BATF-/- and wild-type (WT) CD8+ T cells were mixed in a 1:1 ratio and adoptively transferred into B6.Rag1-/- mice 1 day prior to skin transplantation. Compared with WT CD8+ T cells at the peak of rejection response, BATF-/- CD8+ T cells displayed a dysfunctional phenotype, evident by their failure to differentiate into CD127-KLRG1+ terminal effectors, impaired proliferative capacity and production of pro-inflammatory cytokines/cytotoxic molecules, and diminished capacity to infiltrate allografts. In association with the failure of effector differentiation, BATF-/- CD8+ T cells largely retained TCF1 expression and expressed significantly low levels of T-bet, TOX, and Ki67. At the memory phase, BATF-deficient CD8+ T cells displayed impaired effector differentiation upon allogeneic antigen re-stimulation. Therefore, BATF is a critical transcriptional determinant that governs the terminal differentiation and memory responses of allogeneic CD8+ T cells in the transplantation setting. Targeting BATF in CD8+ T cells may be an attractive therapeutic approach to promote transplant acceptance.
Keywords: BATF; CD8+ T cells; allograft rejection; effector differentiation; memory; transplantation.
Copyright © 2022 Li, Zou, Chen, Cheng, Britz, Weng and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

