Canonical NF-κB p65, but Not p105, Contributes to IL-1β-Induced IL-8 Expression in Cardiac Fibroblasts
- PMID: 35514973
- PMCID: PMC9065446
- DOI: 10.3389/fimmu.2022.863309
Canonical NF-κB p65, but Not p105, Contributes to IL-1β-Induced IL-8 Expression in Cardiac Fibroblasts
Abstract
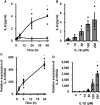
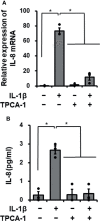
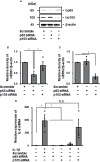
Cardiac fibroblasts participate in the inflammatory process of heart diseases as sentinel cells of the cardiac tissue. In this study, we investigated the effect of the proinflammatory cytokine, interleukin 1β (IL-1β), on the expression of interleukin 8 (IL-8), which contributes to the induction of innate immunity via the activation and recruitment of innate immune cells, such as neutrophils, to the site of inflammation in canine cardiac fibroblasts. IL-1β mediates IL-8 mRNA expression and protein release in a dose- and time-dependent manner. The IL-β-mediated IL-8 protein release and mRNA expression were inhibited by 2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide, an inhibitor of the transcription factor, nuclear factor (NF)-κB. In cells treated with IL-1β, NF-κB p65 and p105 were transiently phosphorylated, indicating the activation of NF-κB. However, IL-1β failed to induce IL-8 mRNA expression in the cells transfected with p65 small interfering RNA (siRNA), but not in those transfected with p105 siRNA. These observations suggest that IL-1β induces IL-8 expression via the activation of NF-κB p65 in canine cardiac fibroblasts.
Keywords: NF-κB p65; cardiac fibroblasts; inflammation; interleukin 1β; interleukin 8.
Copyright © 2022 Mizuno, Nakano, Nose, Matsumura, Nii, Kurogochi, Sugiya and Uechi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

