Influenza Vaccination Results in Differential Hemagglutinin Stalk-Specific Fc-Mediated Functions in Individuals Living With or Without HIV
- PMID: 35514992
- PMCID: PMC9062095
- DOI: 10.3389/fimmu.2022.873191
Influenza Vaccination Results in Differential Hemagglutinin Stalk-Specific Fc-Mediated Functions in Individuals Living With or Without HIV
Abstract
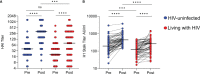
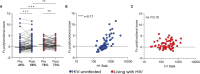
Influenza virus hemagglutinin (HA) stalk-specific antibodies have been shown to potently induce Fc-mediated effector functions which are important in protection from disease. In placebo-controlled maternal influenza (MatFlu) vaccination trials of pregnant women living with or without HIV, reduced risk of influenza illness was associated with high HA stalk antibody titers following trivalent inactivated vaccination (TIV). However, the mechanisms of immunity conferred by the HA stalk antibodies were not well understood. Here, we investigated HA stalk-specific Fc effector functions including antibody-dependent cellular phagocytosis (ADCP), antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent complement deposition (ADCD), and FcγRIIa and FcγRIIIa binding in response to seasonal influenza vaccination. These were measured pre- and 1-month post-vaccination in 141 HIV-uninfected women (67 TIV and 74 placebo recipients) and 119 women living with HIV (WLWH; 66 TIV and 53 placebo recipients). In contrast to HIV-uninfected women, where HA stalk-specific ADCP and FcγRIIa binding were significantly boosted, WLWH showed no increase in response to vaccination. HA stalk-specific ADCC potential and FcγRIIIa binding were not boosted regardless of HIV status but were higher in WLWH compared with HIV-uninfected women prior to vaccination. HA stalk-specific ADCD was significantly increased by vaccination in all women, but was significantly lower in the WLWH both pre- and post- vaccination. Co-ordination between HA stalk-specific ADCP and ADCD in WLWH was improved by vaccination. Fc polyfunctionality was enhanced by vaccination in HIV-uninfected women and driven by the HA stalk antibody titers. However, in the WLWH, higher pre-vaccination Fc polyfunctionality was maintained post-vaccination but was decoupled from titer. Overall, we showed differential regulation of Fc effector HA stalk responses, suggesting that HIV infection results in unique humoral immunity in response to influenza vaccination, with relevance for future strategies that aim to target the HA stalk in this population.
Keywords: Fc effector functions; HIV co-infection; antibody- dependent cellular cytotoxicity (ADCC); antibody-dependent cellular phagocytosis (ADCP); antibody-dependent complement deposition (ADCD); hemagglutinin stalk antibodies; influenza vaccination.
Copyright © 2022 Motsoeneng, Dhar, Nunes, Krammer, Madhi, Moore and Richardson.
Conflict of interest statement
FK reports royalties (Avimex), consulting fees (Pfizer, Seqirus, Third Rock Ventures and Avimex), and payment for academic lectures during the past two years. FK is also named as inventor on IP filed by the Icahn School of Medicine at Mount Sinai for influenza virus vaccines and therapeutics, SARS-CoV-2 vaccines and SARS-CoV-2 serological assays. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Hemagglutinin Stalk-Specific Fc-Mediated Functions Are Associated With Protection Against Influenza Illness After Seasonal Influenza Vaccination.J Infect Dis. 2024 Dec 16;230(6):1329-1336. doi: 10.1093/infdis/jiae241. J Infect Dis. 2024. PMID: 38743692 Free PMC article. Clinical Trial.
-
Antibody Responses with Fc-Mediated Functions after Vaccination of HIV-Infected Subjects with Trivalent Influenza Vaccine.J Virol. 2016 May 27;90(12):5724-5734. doi: 10.1128/JVI.00285-16. Print 2016 Jun 15. J Virol. 2016. PMID: 27053553 Free PMC article.
-
Hemagglutinin Stalk Antibody Responses Following Trivalent Inactivated Influenza Vaccine Immunization of Pregnant Women and Association With Protection From Influenza Virus Illness.Clin Infect Dis. 2020 Aug 14;71(4):1072-1079. doi: 10.1093/cid/ciz927. Clin Infect Dis. 2020. PMID: 31565750 Free PMC article.
-
ADCC: An underappreciated correlate of cross-protection against influenza?Front Immunol. 2023 Feb 22;14:1130725. doi: 10.3389/fimmu.2023.1130725. eCollection 2023. Front Immunol. 2023. PMID: 36911705 Free PMC article. Review.
-
Extra-Neutralizing FcR-Mediated Antibody Functions for a Universal Influenza Vaccine.Front Immunol. 2019 Mar 18;10:440. doi: 10.3389/fimmu.2019.00440. eCollection 2019. Front Immunol. 2019. PMID: 30949165 Free PMC article. Review.
Cited by
-
Hemagglutinin Stalk-Specific Fc-Mediated Functions Are Associated With Protection Against Influenza Illness After Seasonal Influenza Vaccination.J Infect Dis. 2024 Dec 16;230(6):1329-1336. doi: 10.1093/infdis/jiae241. J Infect Dis. 2024. PMID: 38743692 Free PMC article. Clinical Trial.
-
Structure and function of a cross-neutralizing influenza neuraminidase antibody that accommodates recent N2 NA Asn245 glycosylation.bioRxiv [Preprint]. 2025 Jul 1:2025.06.30.662356. doi: 10.1101/2025.06.30.662356. bioRxiv. 2025. PMID: 40631320 Free PMC article. Preprint.
-
Fc-Effector-Independent in vivo Activity of a Potent Influenza B Neuraminidase Broadly Neutralizing Antibody.Viruses. 2023 Jul 13;15(7):1540. doi: 10.3390/v15071540. Viruses. 2023. PMID: 37515226 Free PMC article.
-
Despite delayed kinetics, people living with HIV achieve equivalent antibody function after SARS-CoV-2 infection or vaccination.Front Immunol. 2023 Aug 3;14:1231276. doi: 10.3389/fimmu.2023.1231276. eCollection 2023. Front Immunol. 2023. PMID: 37600825 Free PMC article.
-
HIV-related immune activation attenuates polyfunctional IgG and memory B-cell responses to Tdap immunization during pregnancy.EBioMedicine. 2024 Jun;104:105179. doi: 10.1016/j.ebiom.2024.105179. Epub 2024 Jun 7. EBioMedicine. 2024. PMID: 38848615 Free PMC article.
References
-
- Tempia S, Walaza S, Moyes J, Cohen AL, McMorrow ML, Treurnicht FK, et al. . Quantifying How Different Clinical Presentations, Levels of Severity, and Healthcare Attendance Shape the Burden of Influenza-Associated Illness: A Modeling Study From South Africa. Clin Infect Dis (2019) 69:1036–48. doi: 10.1093/CID/CIY1017 - DOI - PMC - PubMed
-
- Madhi SA, Maskew M, Koen A, Kuwanda L, Besselaar TG, Naidoo D, et al. . Trivalent Inactivated Influenza Vaccine in African Adults Infected With Human Immunodeficient Virus: Double Blind, Randomized Clinical Trial of Efficacy, Immunogenicity, and Safety. Clin Infect Dis (2011) 52:128–37. doi: 10.1093/CID/CIQ004 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

