Anti-platelet factor 4 immunoglobulin G levels in vaccine-induced immune thrombocytopenia and thrombosis: Persistent positivity through 7 months
- PMID: 35515079
- PMCID: PMC9066380
- DOI: 10.1002/rth2.12707
Anti-platelet factor 4 immunoglobulin G levels in vaccine-induced immune thrombocytopenia and thrombosis: Persistent positivity through 7 months
Abstract
Background: Anti-platelet factor 4 (PF4) antibodies that activate platelets via FcγRIIA drive the pathophysiology of vaccine-induced immune thrombocytopenia and thrombosis (VITT). Evolution of these antibodies and their ability to activate platelets after initial treatment remains unknown.
Objectives: To assess how clinical and platelet parameters, anti-PF4 antibody levels, and patient serum reactivity changes during follow-up after VITT presentation.
Methods: We describe cases of seven discharged VITT patients that were followed from diagnosis up to 280 days (range 199-280) after vaccination. We measured anti-PF4 antibodies and PF4 levels in patient serum during follow-up and tested the ability of patient serum to activate healthy donor platelets and patient platelets over time.
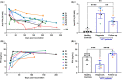
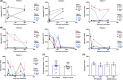
Results: Anti-PF4 immunoglobulin G antibody levels are very high at diagnosis (0.9-2.6 OD) and remain relatively high (>1.0 OD) in all patients, except one treated with rituximab, at 7 months post vaccination. All patients were on direct oral anticoagulants throughout follow-up and no patients had recurrent thrombosis. Patients' platelets during follow-up have normal FcγRIIA levels and responsiveness to platelet agonists. Patient diagnostic serum strongly activated control platelets, either alone or with PF4. Most follow-up serum alone was weaker at stimulating donor and patient platelets. However, follow-up serum beyond 150 days still strongly activated platelets with PF4 addition in three patients. Patient serum PF4 levels were lower than controls at diagnosis but returned within normal range by day 50.
Conclusions: Explanations for reduced platelet activation during follow-up, despite similar total anti-PF4 antibody levels, remains unclear. Clinical implications of persistent anti-PF4 antibodies in VITT require further study.
Keywords: AZD1222 vaccination; FcγRIIA; VITT; anti‐PF4 antibodies; thrombosis.
© 2022 The Authors. Research and Practice in Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis (ISTH).
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

