A dendritic, redox-responsive, supramolecular (Dr.S) system for lysis-triggered delivery for drug-resistant renal cancer
- PMID: 35515145
- PMCID: PMC9057208
- DOI: 10.1039/d0ra06444k
A dendritic, redox-responsive, supramolecular (Dr.S) system for lysis-triggered delivery for drug-resistant renal cancer
Abstract
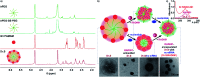
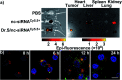
Purpose: Aiming to improve the drug loading capacity of dendritic nanoparticles and enhance delivery efficacy in drug-resistant cancer, we developed and optimized a more advanced dendritic, redox-responsive, supramolecular (Dr.S) system for intravenous RAD001 administration. Materials and methods: The Dr.S system was engineered by linking 3rd generation polyamidoamine dendrimers (G3 PAMAM) with 8-arm polyethylene glycol (PEG) to encapsulate a molecular targeted agent RAD001. The drug-loading capacity was measured by ultraviolet-visible spectrophotometry. In vitro release behavior was determined with a two-compartment model, and the in vivo distribution pattern was tracked by Cy5.5 fluorescence. The therapeutic effect of Dr.S/RAD001 was evaluated in RAD001-resistant cancer cells and tumor-bearing nude mice, respectively. Results: The Dr.S system encapsulating RAD001 with a loading efficiency of 10.6% formed a core-shell structure, by shifting hydrophobic PAMAM/RAD001 components towards inner space and exposing the hydrophilic PEG on the surface. The Dr.S/RAD001 system could respond to a lysis-mimicking reduction stimulus, and functionally release cargoes to facilitate tumor accumulation and cellular internalization. These features contributed to the enhanced anti-tumor activity of RAD001 in renal cancers in vitro and in vivo. The Dr.S/RAD001 system also reversed acquired RAD001-resistance by a 60-fold increase in tumor accumulation of the therapeutics. Conclusion: The functional Dr.S/RAD001 system enables lysis-triggered release of RAD001 to achieve better tumor accumulation, which helps overcome acquired drug resistance in renal cancers.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures
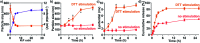


Similar articles
-
Redox and pH dual responsive poly(amidoamine) dendrimer-poly(ethylene glycol) conjugates for intracellular delivery of doxorubicin.Acta Biomater. 2016 May;36:241-53. doi: 10.1016/j.actbio.2016.03.027. Epub 2016 Mar 16. Acta Biomater. 2016. PMID: 26995505
-
Transferrin Conjugated pH- and Redox-Responsive Poly(Amidoamine) Dendrimer Conjugate as an Efficient Drug Delivery Carrier for Cancer Therapy.Int J Nanomedicine. 2020 Apr 22;15:2751-2764. doi: 10.2147/IJN.S238536. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32368053 Free PMC article.
-
cRGD mediated redox and pH dual responsive poly(amidoamine) dendrimer-poly(ethylene glycol) conjugates for efficiently intracellular antitumor drug delivery.Colloids Surf B Biointerfaces. 2020 Oct;194:111195. doi: 10.1016/j.colsurfb.2020.111195. Epub 2020 Jun 12. Colloids Surf B Biointerfaces. 2020. PMID: 32619785
-
Dendritic nanoparticles for cutaneous drug delivery--testing in human skin and reconstructed human skin.Curr Pharm Des. 2015;21(20):2784-800. doi: 10.2174/1381612821666150428142515. Curr Pharm Des. 2015. PMID: 25925118 Review.
-
Future directions in the treatment of hormone-sensitive advanced breast cancer: the RAD001 (Everolimus)-letrozole clinical program.Semin Oncol. 2006 Apr;33(2 Suppl 7):S18-25. doi: 10.1053/j.seminoncol.2006.03.024. Semin Oncol. 2006. PMID: 16730273 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

