Ligand exchange processes between molybdenum and zinc additives in lubricants: evidence from NMR (1H, 13C, 31P) and HPLC-MS analysis
- PMID: 35515166
- PMCID: PMC9057216
- DOI: 10.1039/d0ra07329f
Ligand exchange processes between molybdenum and zinc additives in lubricants: evidence from NMR (1H, 13C, 31P) and HPLC-MS analysis
Abstract
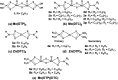
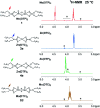
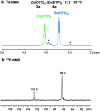
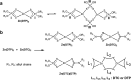
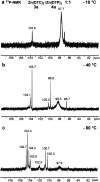
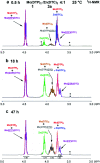
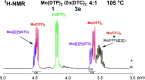
The tribological performances of engine oils have been shown to be enhanced by the synergistic interactions between Mo dithiocarbamates (Mo(DTC)2) with other additives, and notably Zn dithiophosphates (Zn(DTP)2). Being two key components in formulated lubricants, a detailed understanding of the mechanisms involved between these two types of additives is needed to develop engine oils with enhanced friction reduction performances, and improved fuel economy. In this context, we report here the investigation at the molecular level of the interactions between Mo and Zn complexes with DTC and DTP ligands using laboratory experiments. Our analytical approach comprised NMR spectroscopy (1H, 13C, 31P) allowing direct investigation of both homoleptic and heteroleptic Mo and Zn complexes as well as a specifically-developed HPLC-MS method for the investigation of the different DTC species formed during lubricant ageing experiments. The results showed that ligand exchange reactions between Mo(DTP)2 and Zn(DTC)2 complexes strongly favor the migration of the DTC ligands from Zn to Mo, illustrating the higher affinity of Mo for DTC ligands. In the case of binary mixtures involving Mo(DTC)2 and Zn(DTP)2 - a combination of additives frequently used in formulated lubricants - the formation of mixed complexes (Mo(DTC)(DTP)) resulting from ligand exchange reactions could be directly evidenced for the first time by the analytical methods used. These species could account, at least to some extent, for the synergistic effect of Mo(DTC)2 and Zn(DTP)2 on the friction reducing properties of engine oils. However, they were formed in significantly lower proportions than those previously reported in the literature using indirect methods.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
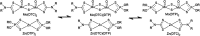



References
-
- Spikes H. A. Friction modifier additives. Tribol. Lett. 2015;60:5. doi: 10.1007/s11249-015-0589-z. - DOI
-
- De Barros Bouchet M. I. Martina J. M. Le Mogne Th. Bilasa P. Vachera B. Yamada Y. Mechanisms of MoS2 formation by MoDTC in presence of ZnDTP: effect of oxidative degradation. Wear. 2005;258:1643–1650. doi: 10.1016/j.wear.2004.11.019. - DOI
-
- Graham J. Spikes H. Jensen R. The friction reducing properties of molybdenum dialkyldithiocarbamate additives: Part II - durability of friction reducing capability. Tribol. Lett. 2001;44:637–647.
-
- Inoue K. Tominaga E. Akiyama K. Ashida T. Effects of lubricant composition on fuel efficiency in modern engines. SAE Trans. 1995;104:728–736.
-
- Graham J. Spikes H. Korcek S. The friction reducing properties of molybdenum dialkyldithiocarbamate additives: Part I – factors influencing friction reduction. Tribol. Lett. 2001;44:626–636.
LinkOut - more resources
Full Text Sources
Other Literature Sources

