Assessing the compatibility of primary human hepatocyte culture within porous silk sponges
- PMID: 35515172
- PMCID: PMC9057238
- DOI: 10.1039/d0ra04954a
Assessing the compatibility of primary human hepatocyte culture within porous silk sponges
Abstract
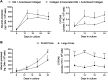
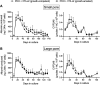
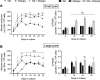
Donor organ shortages have prompted the development of alternative implantable human liver tissues for patients suffering from end-stage liver failure. Purified silk proteins provide desirable features for generating implantable tissues, including sustainable sourcing from insects/arachnids, biocompatibility, tunable mechanical properties and degradation rates, and low immunogenicity upon implantation. While different cell types were previously cultured for weeks within silk-based scaffolds, it remains unclear whether such scaffolds can be used to culture primary human hepatocytes (PHH) isolated from livers. Therefore, here we assessed the compatibility of PHH culture within porous silk scaffolds that enable diffusion of oxygen/nutrients through the pores. We found that incorporation of type I collagen during the fabrication and/or autoclaving of porous silk scaffolds, as opposed to simple adsorption of collagen onto pre-fabricated silk scaffolds, was necessary to enable robust PHH attachment/function. Scaffolds with small pores (73 ± 25 μm) promoted larger PHH spheroids and consequently higher PHH functions than large pores (235 ± 84 μm) for at least 1 month in culture. Further incorporation of supportive fibroblasts into scaffolds enhanced PHH functions up to 5-fold relative to scaffolds with PHHs alone and 2D co-cultures on plastic. Lastly, encapsulating PHHs within protein hydrogels while housed in the silk scaffold led to higher functions than protein hydrogel-only or silk-only controls. In conclusion, porous silk scaffolds containing extracellular matrix proteins can be used for the culture of PHHs ± supportive non-parenchymal cells, which can be further built on in the future to create optimized silk-based liver tissue surrogates for cell-based therapy.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures



Similar articles
-
Functional hepatocyte clusters on bioactive blend silk matrices towards generating bioartificial liver constructs.Acta Biomater. 2018 Feb;67:167-182. doi: 10.1016/j.actbio.2017.11.053. Epub 2017 Dec 6. Acta Biomater. 2018. PMID: 29223705
-
Physiologically inspired culture medium prolongs the lifetime and insulin sensitivity of human hepatocytes in micropatterned co-cultures.Toxicology. 2021 Feb 15;449:152662. doi: 10.1016/j.tox.2020.152662. Epub 2020 Dec 24. Toxicology. 2021. PMID: 33359713 Free PMC article.
-
Microscale Collagen and Fibroblast Interactions Enhance Primary Human Hepatocyte Functions in Three-Dimensional Models.Gene Expr. 2020 Jun 12;20(1):1-18. doi: 10.3727/105221620X15868728381608. Epub 2020 Apr 14. Gene Expr. 2020. PMID: 32290899 Free PMC article.
-
Upgrading Hepatic Differentiation and Functions on 3D Printed Silk-Decellularized Liver Hybrid Scaffolds.ACS Biomater Sci Eng. 2021 Aug 9;7(8):3861-3873. doi: 10.1021/acsbiomaterials.1c00671. Epub 2021 Jul 28. ACS Biomater Sci Eng. 2021. PMID: 34318665
-
Development of bilayered porous silk scaffolds for thymus bioengineering.Biomater Adv. 2023 Apr;147:213320. doi: 10.1016/j.bioadv.2023.213320. Epub 2023 Jan 31. Biomater Adv. 2023. PMID: 36739783
Cited by
-
Preparation and characterization of propolis reinforced eggshell membrane/ GelMA composite hydrogel for biomedical applications.BMC Biotechnol. 2023 Jul 11;23(1):21. doi: 10.1186/s12896-023-00788-4. BMC Biotechnol. 2023. PMID: 37434201 Free PMC article.
-
Natural Scaffolds Used for Liver Regeneration: A Narrative Update.Stem Cell Rev Rep. 2022 Oct;18(7):2262-2278. doi: 10.1007/s12015-022-10362-8. Epub 2022 Mar 23. Stem Cell Rev Rep. 2022. PMID: 35320512 Review.
References
-
- Murray C. J. et al., Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. doi: 10.1016/S0140-6736(12)61689-4. - DOI - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

