Rh(iii)-catalyzed C-7 arylation of indolines with arylsilanes via C-H activation
- PMID: 35515217
- PMCID: PMC9064675
- DOI: 10.1039/c9ra04142g
Rh(iii)-catalyzed C-7 arylation of indolines with arylsilanes via C-H activation
Abstract
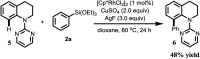
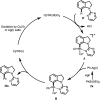
Site-selective synthesis of C-7 arylated indolines has been achieved via oxidative arylation of indolines with arylsilanes under Rh(iii)-catalyzed C-H activation of indolines by using CuSO4 as a co-oxidant. This transformation has been explored for a wide range of substrates under mild conditions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

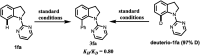
Similar articles
-
Access to Six- and Seven-Membered 1,7-Fused Indolines via Rh(III)-Catalyzed Redox-Neutral C7-Selective C-H Functionalization of Indolines with Alkynes and Alkenes.J Org Chem. 2015 Jun 19;80(12):6238-49. doi: 10.1021/acs.joc.5b00684. Epub 2015 Jun 1. J Org Chem. 2015. PMID: 25989195
-
Rhodium(III)-Catalyzed Direct C-H Arylation of Various Acyclic Enamides with Arylsilanes.Org Lett. 2021 Jan 1;23(1):31-36. doi: 10.1021/acs.orglett.0c03578. Epub 2020 Dec 18. Org Lett. 2021. PMID: 33337165
-
Rhodium-Catalyzed C-H Arylation of Indoles with Arylsilanes at Room Temperature.J Org Chem. 2024 Nov 15;89(22):16406-16418. doi: 10.1021/acs.joc.4c01549. Epub 2024 Nov 6. J Org Chem. 2024. PMID: 39504433
-
Recent advances in rhodium-catalyzed C(sp2)-H (hetero)arylation.Org Biomol Chem. 2021 Oct 14;19(39):8442-8465. doi: 10.1039/d1ob01190a. Org Biomol Chem. 2021. PMID: 34553744 Review.
-
Co(III), Rh(III) & Ir(III)-Catalyzed Direct C-H Alkylation/Alkenylation/Arylation with Carbene Precursors.Chem Asian J. 2021 Mar 1;16(5):443-459. doi: 10.1002/asia.202001219. Epub 2021 Feb 4. Chem Asian J. 2021. PMID: 33448653 Review.
Cited by
-
Regioselective Arylation of Quinoline N-Oxides (C8), Indolines (C7) and N-tert-Butylbenzamide with Arylboronic Acids.ACS Omega. 2019 Dec 27;5(1):904-913. doi: 10.1021/acsomega.9b03884. eCollection 2020 Jan 14. ACS Omega. 2019. PMID: 31956844 Free PMC article.
-
Cu-Catalyzed Arylation of Bromo-Difluoro-Acetamides by Aryl Boronic Acids, Aryl Trialkoxysilanes and Dimethyl-Aryl-Sulfonium Salts: New Entries to Aromatic Amides.Molecules. 2021 May 16;26(10):2957. doi: 10.3390/molecules26102957. Molecules. 2021. PMID: 34065691 Free PMC article.
-
Catalytic Atroposelective C7 Functionalisation of Indolines and Indoles.Chemistry. 2022 Jan 3;28(1):e202103365. doi: 10.1002/chem.202103365. Epub 2021 Nov 5. Chemistry. 2022. PMID: 34676929 Free PMC article.
References
-
- Mason J. S. Morize I. Menard P. R. Cheney D. L. Hulme C. Labaudiniere R. F. J. Med. Chem. 1999;42:3251. doi: 10.1021/jm9806998. - DOI - PubMed
- Nicolaou K. C. Pfefferkorn J. A. Roecker A. J. Cao G. Barluenga S. Mitchell H. J. J. Am. Chem. Soc. 2000;122:9939. doi: 10.1021/ja002033k. - DOI
- Podoll J. D. Liu Y. Chang L. Walls S. Wang W. Wang X. Proc. Natl. Acad. Sci. U. S. A. 2013;110:15573. doi: 10.1073/pnas.1310459110. - DOI - PMC - PubMed
-
- Pindur U. Lemster T. Curr. Med. Chem. 2001;8:1681. doi: 10.2174/0929867013371941. - DOI - PubMed
- Knçlker H. Reddy K. R. Chem. Rev. 2002;102:4303. doi: 10.1021/cr020059j. - DOI - PubMed
- Horton D. A. Bourne G. T. Smythe M. L. Chem. Rev. 2003;103:893. doi: 10.1021/cr020033s. - DOI - PubMed
- Kochanowska-Karamyan A. J. Hamann M. T. Chem. Rev. 2010;110:4489. doi: 10.1021/cr900211p. - DOI - PMC - PubMed
- Bell M. G. Gernert D. L. Grese T. A. Belvo M. D. Borromeo P. S. Kelley S. A. Kennedy J. H. Kolis S. P. Lander P. A. Richey R. J. Med. Chem. 2007;50:6443. doi: 10.1021/jm701186z. - DOI - PubMed
- Owa T. Yokoi A. Yamazaki K. Yoshimatsu K. Yamori T. Nagasu T. J. Med. Chem. 2002;45:4913. doi: 10.1021/jm0201060. - DOI - PubMed
-
-
For selected reviews on C–H bond functionalization, see:
- Sambiagio C. Schönbauer D. Blieck R. Dao-Huy T. Pototschnig G. Schaaf P. Wiesinger T. Zia M. F. Wencel-Delord J. Besset T. Maesa B. U. W. Schnürch M. Chem. Soc. Rev. 2018;47:6603. doi: 10.1039/C8CS00201K. - DOI - PMC - PubMed
- He J. Wasa M. Chan K. S. L. Shao Q. Yu J.-Q. Chem. Rev. 2017;117:8754. doi: 10.1021/acs.chemrev.6b00622. - DOI - PMC - PubMed
- Wei Y. Hu P. Zhang M. Su W. Chem. Rev. 2017;117:8864. doi: 10.1021/acs.chemrev.6b00516. - DOI - PubMed
- Wencel-Delord J. Drçge T. Kiu F. Glorius F. Chem. Soc. Rev. 2011;40:4740. doi: 10.1039/C1CS15083A. - DOI - PubMed
- Kuhl N. Hopkinson M. N. Wencel-Delord J. Glorius F. Angew. Chem. 2012;124:10382. doi: 10.1002/ange.201203269. - DOI - PubMed
- Mousseau J. J. Charette A. B. Acc. Chem. Res. 2013;46:412. doi: 10.1021/ar300185z. - DOI - PubMed
-
-
-
For selected reviews, see:
- Seregin I. V. Gevorgyan V. Chem. Soc. Rev. 2007;36:1173. doi: 10.1039/B606984N. - DOI - PMC - PubMed
- Joucla L. Djakovitch L. Adv. Synth. Catal. 2009;351:673. doi: 10.1002/adsc.200900059. - DOI
- Beck E. M. Gaunt M. J. Top. Curr. Chem. 2010;292:85. doi: 10.1007/128_2009_15. - DOI - PubMed
- Cacchi S. Fabrizi G. Chem. Rev. 2011;111:PR215. doi: 10.1021/cr100403z. - DOI - PubMed
-
-
- Robbins D. W. Boebel T. A. Hartwig J. F. J. Am. Chem. Soc. 2010;132:4068. doi: 10.1021/ja1006405. - DOI - PubMed
- Yang Y. Qiu X. Zhao Y. Mu Y. Shi Z. J. Am. Chem. Soc. 2016;138:495. doi: 10.1021/jacs.5b11569. - DOI - PubMed
- Xu L. Zhang C. He Y. Tan L. Ma D. Angew. Chem., Int. Ed. 2016;55:321. doi: 10.1002/anie.201508117. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

