Rh(iii)-catalyzed C-7 arylation of indolines with arylsilanes via C-H activation
- PMID: 35515217
- PMCID: PMC9064675
- DOI: 10.1039/c9ra04142g
Rh(iii)-catalyzed C-7 arylation of indolines with arylsilanes via C-H activation
Abstract
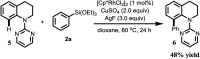
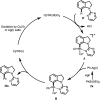
Site-selective synthesis of C-7 arylated indolines has been achieved via oxidative arylation of indolines with arylsilanes under Rh(iii)-catalyzed C-H activation of indolines by using CuSO4 as a co-oxidant. This transformation has been explored for a wide range of substrates under mild conditions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

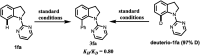
References
-
- Mason J. S. Morize I. Menard P. R. Cheney D. L. Hulme C. Labaudiniere R. F. J. Med. Chem. 1999;42:3251. doi: 10.1021/jm9806998. - DOI - PubMed
- Nicolaou K. C. Pfefferkorn J. A. Roecker A. J. Cao G. Barluenga S. Mitchell H. J. J. Am. Chem. Soc. 2000;122:9939. doi: 10.1021/ja002033k. - DOI
- Podoll J. D. Liu Y. Chang L. Walls S. Wang W. Wang X. Proc. Natl. Acad. Sci. U. S. A. 2013;110:15573. doi: 10.1073/pnas.1310459110. - DOI - PMC - PubMed
-
- Pindur U. Lemster T. Curr. Med. Chem. 2001;8:1681. doi: 10.2174/0929867013371941. - DOI - PubMed
- Knçlker H. Reddy K. R. Chem. Rev. 2002;102:4303. doi: 10.1021/cr020059j. - DOI - PubMed
- Horton D. A. Bourne G. T. Smythe M. L. Chem. Rev. 2003;103:893. doi: 10.1021/cr020033s. - DOI - PubMed
- Kochanowska-Karamyan A. J. Hamann M. T. Chem. Rev. 2010;110:4489. doi: 10.1021/cr900211p. - DOI - PMC - PubMed
- Bell M. G. Gernert D. L. Grese T. A. Belvo M. D. Borromeo P. S. Kelley S. A. Kennedy J. H. Kolis S. P. Lander P. A. Richey R. J. Med. Chem. 2007;50:6443. doi: 10.1021/jm701186z. - DOI - PubMed
- Owa T. Yokoi A. Yamazaki K. Yoshimatsu K. Yamori T. Nagasu T. J. Med. Chem. 2002;45:4913. doi: 10.1021/jm0201060. - DOI - PubMed
-
-
For selected reviews on C–H bond functionalization, see:
- Sambiagio C. Schönbauer D. Blieck R. Dao-Huy T. Pototschnig G. Schaaf P. Wiesinger T. Zia M. F. Wencel-Delord J. Besset T. Maesa B. U. W. Schnürch M. Chem. Soc. Rev. 2018;47:6603. doi: 10.1039/C8CS00201K. - DOI - PMC - PubMed
- He J. Wasa M. Chan K. S. L. Shao Q. Yu J.-Q. Chem. Rev. 2017;117:8754. doi: 10.1021/acs.chemrev.6b00622. - DOI - PMC - PubMed
- Wei Y. Hu P. Zhang M. Su W. Chem. Rev. 2017;117:8864. doi: 10.1021/acs.chemrev.6b00516. - DOI - PubMed
- Wencel-Delord J. Drçge T. Kiu F. Glorius F. Chem. Soc. Rev. 2011;40:4740. doi: 10.1039/C1CS15083A. - DOI - PubMed
- Kuhl N. Hopkinson M. N. Wencel-Delord J. Glorius F. Angew. Chem. 2012;124:10382. doi: 10.1002/ange.201203269. - DOI - PubMed
- Mousseau J. J. Charette A. B. Acc. Chem. Res. 2013;46:412. doi: 10.1021/ar300185z. - DOI - PubMed
-
-
-
For selected reviews, see:
- Seregin I. V. Gevorgyan V. Chem. Soc. Rev. 2007;36:1173. doi: 10.1039/B606984N. - DOI - PMC - PubMed
- Joucla L. Djakovitch L. Adv. Synth. Catal. 2009;351:673. doi: 10.1002/adsc.200900059. - DOI
- Beck E. M. Gaunt M. J. Top. Curr. Chem. 2010;292:85. doi: 10.1007/128_2009_15. - DOI - PubMed
- Cacchi S. Fabrizi G. Chem. Rev. 2011;111:PR215. doi: 10.1021/cr100403z. - DOI - PubMed
-
-
- Robbins D. W. Boebel T. A. Hartwig J. F. J. Am. Chem. Soc. 2010;132:4068. doi: 10.1021/ja1006405. - DOI - PubMed
- Yang Y. Qiu X. Zhao Y. Mu Y. Shi Z. J. Am. Chem. Soc. 2016;138:495. doi: 10.1021/jacs.5b11569. - DOI - PubMed
- Xu L. Zhang C. He Y. Tan L. Ma D. Angew. Chem., Int. Ed. 2016;55:321. doi: 10.1002/anie.201508117. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

