Rational design of a polypyrrole-based competent bifunctional magnetic nanocatalyst
- PMID: 35515252
- PMCID: PMC9064774
- DOI: 10.1039/c9ra02544h
Rational design of a polypyrrole-based competent bifunctional magnetic nanocatalyst
Abstract
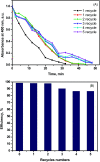
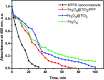
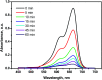
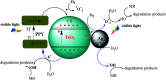
The combination of conducting polymers with semiconductors for the fabrication of organic/inorganic hybrid nanocatalysts is one of the most promising research areas for many applications. In this work, the synthesized nanocomposite combines several advantages such as the photoresponse shift from the UV region toward visible light by narrowing the band gap of the semiconductor, magnetic separation ability and dual applications including the catalytic reduction of p-nitrophenol (PNP) and the photocatalytic degradation of methylene blue (MB) dye. In addition to the core magnetite nanoparticles (NPs), the synthesized nanocomposite contains polypyrrole (PPY) and TiO2 shells that are decorated with silver metal NPs to prevent electron-hole recombination and to enhance the catalytic performance. Indeed, the catalytic PNP reduction experiments reveal that the synthesized nanocomposite exhibits significantly high catalytic activity with a rate constant of 0.1169 min-1. Moreover, the photocatalytic experiments show that the synthesized nanophotocatalyst has a boosting effect toward MB dye degradation under normal daytime visible light irradiation with a rate constant of 6.38 × 10-2 min-1. The synergetic effect between silver NPs, PPY and TiO2 is thought to play a fundamental role in enhancing the photocatalytic activity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Li J. Dong X. a. Zhang G. Cui W. Cen W. Wu Z. Lee S. Dong F. J. Mater. Chem. A. 2019;7:3366–3374. doi: 10.1039/C8TA11627J. - DOI
-
- Li X. Zhang W. Cui W. Li J. Sun Y. Jiang G. Huang H. Zhang Y. Dong F. Chem. Eng. J. 2019;370:1366–1375. doi: 10.1016/j.cej.2019.04.003. - DOI
-
- Du Y. Chen H. Chen R. Xu N. Appl. Catal., A. 2004;277:259–264. doi: 10.1016/j.apcata.2004.09.018. - DOI
-
- Deng F. Li Y. Luo X. Yang L. Tu X. Colloids Surf., A. 2012;395:183–189. doi: 10.1016/j.colsurfa.2011.12.029. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

