Structural exploration of rhodium catalysts and their kinetic studies for efficient parahydrogen-induced polarization by side arm hydrogenation
- PMID: 35515260
- PMCID: PMC9064692
- DOI: 10.1039/c9ra02580d
Structural exploration of rhodium catalysts and their kinetic studies for efficient parahydrogen-induced polarization by side arm hydrogenation
Abstract
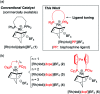
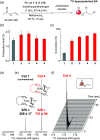
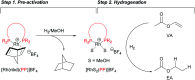
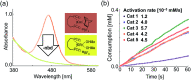
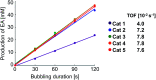
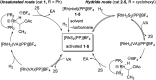
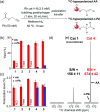
Parahydrogen-induced polarization (PHIP) is a rapid and cost-effective hyperpolarization technique using transition metal-catalysed hydrogenation with parahydrogen. We examined rhodium catalysts and their kinetic studies, rarely considered in the research of current PHIP. It emerged that rhodium complexes with electron-donating bisphosphine ligands, with a dicyclohexylphosphino group, appear to be more effective than conventional rhodium catalysts.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Tailoring rhodium-based metal-organic layers for parahydrogen-induced polarization: achieving 20% polarization of 1H in liquid phase.Natl Sci Rev. 2024 Nov 13;12(1):nwae406. doi: 10.1093/nsr/nwae406. eCollection 2025 Jan. Natl Sci Rev. 2024. PMID: 39764503 Free PMC article.
-
More Than 12 % Polarization and 20 Minute Lifetime of 15 N in a Choline Derivative Utilizing Parahydrogen and a Rhodium Nanocatalyst in Water.Angew Chem Int Ed Engl. 2018 Aug 13;57(33):10692-10696. doi: 10.1002/anie.201804185. Epub 2018 Jul 9. Angew Chem Int Ed Engl. 2018. PMID: 29923285
-
Parahydrogen-Induced Polarization Study of the Silica-Supported Vanadium Oxo Organometallic Catalyst.J Phys Chem C Nanomater Interfaces. 2018 Mar 8;122(9):4891-4900. doi: 10.1021/acs.jpcc.7b12069. Epub 2018 Feb 15. J Phys Chem C Nanomater Interfaces. 2018. PMID: 30258526 Free PMC article.
-
Unconventional Parahydrogen-Induced Hyperpolarization Effects in Chemistry and Catalysis: From Photoreactions to Enzymes.ACS Catal. 2025 Apr 4;15(8):6386-6409. doi: 10.1021/acscatal.4c07870. eCollection 2025 Apr 18. ACS Catal. 2025. PMID: 40270879 Free PMC article. Review.
-
Heterogeneous Catalysis and Parahydrogen-Induced Polarization.Chemphyschem. 2021 Jul 16;22(14):1421-1440. doi: 10.1002/cphc.202100153. Epub 2021 Jun 26. Chemphyschem. 2021. PMID: 33969590 Review.
Cited by
-
Tailoring rhodium-based metal-organic layers for parahydrogen-induced polarization: achieving 20% polarization of 1H in liquid phase.Natl Sci Rev. 2024 Nov 13;12(1):nwae406. doi: 10.1093/nsr/nwae406. eCollection 2025 Jan. Natl Sci Rev. 2024. PMID: 39764503 Free PMC article.
-
Advancing homogeneous catalysis for parahydrogen-derived hyperpolarisation and its NMR applications.Chem Sci. 2022 Mar 22;13(17):4670-4696. doi: 10.1039/d2sc00737a. eCollection 2022 May 4. Chem Sci. 2022. PMID: 35655870 Free PMC article. Review.
-
Hydrogenative-PHIP polarized metabolites for biological studies.MAGMA. 2021 Feb;34(1):25-47. doi: 10.1007/s10334-020-00904-x. Epub 2021 Feb 2. MAGMA. 2021. PMID: 33527252 Free PMC article. Review.
-
StereoPHIP: Stereoselective Parahydrogen-Induced Polarization.Angew Chem Int Ed Engl. 2023 Nov 13;62(46):e202311669. doi: 10.1002/anie.202311669. Epub 2023 Sep 26. Angew Chem Int Ed Engl. 2023. PMID: 37714818 Free PMC article.
-
Real-Time Pyruvate Chemical Conversion Monitoring Enabled by PHIP.J Am Chem Soc. 2023 Mar 15;145(10):5864-5871. doi: 10.1021/jacs.2c13198. Epub 2023 Mar 1. J Am Chem Soc. 2023. PMID: 36857108 Free PMC article.
References
-
- Pravica M. G. Weitekamp D. P. Chem. Phys. Lett. 1988;145:255. doi: 10.1016/0009-2614(88)80002-2. - DOI
- Bowers C. R. Weitekamp D. P. J. Am. Chem. Soc. 1987;109:5541. doi: 10.1021/ja00252a049. - DOI
- Eisenschmid T. C. Kirss R. U. Deutsch P. P. Hommeltoft S. I. Eisenberg R. J. Am. Chem. Soc. 1987;109:8089. doi: 10.1021/ja00260a026. - DOI
- Bowers C. R. Weitekamp D. P. Chem. Phys. Lett. 1986;57:2645. - PubMed
-
-
Recent efforts for designing long T1 structures, see for example:
- Imakura Y. Nonaka H. Takakusagi Y. Ichikawa K. Maptue N. R. Funk A. M. Khemtong C. Sando S. Chem.–Asian J. 2018;13:280. doi: 10.1002/asia.201701652. - DOI - PMC - PubMed
- Nonaka H. Hirano M. Imakura Y. Takakusagi Y. Ichikawa K. Sando S. Sci. Rep. 2017;7:40104. doi: 10.1038/srep40104. - DOI - PMC - PubMed
- Nonaka H. Hata R. Doura T. Nishihara T. Kumagai K. Akakabe M. Tsuda M. Ichikawa K. Sando S. Nat. Commun. 2013;4:2411. doi: 10.1038/ncomms3411. - DOI - PMC - PubMed
- Doura T. Hata R. Nonaka H. Ichikawa K. Sando S. Angew. Chem., Int. Ed. 2012;51:10114. doi: 10.1002/anie.201202885. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources
Miscellaneous

