Injectable microfluidic hydrogel microspheres based on chitosan and poly(ethylene glycol) diacrylate (PEGDA) as chondrocyte carriers
- PMID: 35515410
- PMCID: PMC9057443
- DOI: 10.1039/d0ra07318k
Injectable microfluidic hydrogel microspheres based on chitosan and poly(ethylene glycol) diacrylate (PEGDA) as chondrocyte carriers
Abstract
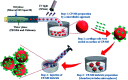
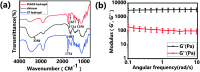
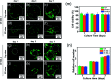
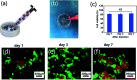
Direct injection of chondrocytes in a minimally invasive way has been regarded as the significantly potential treatment for cartilage repair due to their ability to fill various irregular chondral defects. However, the low cell retention and survival after injection still limited their application in clinical transformation. Herein, we present chondrocyte-laden microspheres as cell carriers based on a double-network hydrogel by the combination of the chitosan and poly(ethylene glycol) diacrylate (PEGDA). The microfluidic technique was applied to prepare size-controllable chitosan/PEGDA hydrogel microspheres (CP-MSs) via the water-in-oil approach after photo-crosslinking and physical-crosslinking. The chondrocytes were laden on CP-MSs, which showed good cell viability and proliferation after long-term cell cultivation. The in vitro investigation further demonstrated that chondrocyte-laden CP-MSs were injectable and the cell viability was still high after injection. In particular, these cell-laden microspheres were self-assembled into a 3D cartilage-like scaffold by a bottom-up strategy based on cell-cell interconnectivity, which suggested that these injectable chondrocyte-laden microspheres showed potential applications as chondrocyte carriers for bottom-to-up cartilage tissue engineering.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
OA cartilage derived chondrocytes encapsulated in poly(ethylene glycol) diacrylate (PEGDA) for the evaluation of cartilage restoration and apoptosis in an in vitro model.Histol Histopathol. 2011 Oct;26(10):1265-78. doi: 10.14670/HH-26.1265. Histol Histopathol. 2011. PMID: 21870330
-
Performance optimization of injectable chitosan hydrogel by combining physical and chemical triple crosslinking structure.J Biomed Mater Res A. 2013 Mar;101(3):684-93. doi: 10.1002/jbm.a.34364. Epub 2012 Aug 31. J Biomed Mater Res A. 2013. PMID: 22941894
-
An in vitro and in vivo comparison of cartilage growth in chondrocyte-laden matrix metalloproteinase-sensitive poly(ethylene glycol) hydrogels with localized transforming growth factor β3.Acta Biomater. 2019 Jul 15;93:97-110. doi: 10.1016/j.actbio.2019.03.046. Epub 2019 Mar 23. Acta Biomater. 2019. PMID: 30914256 Free PMC article.
-
New perspectives in the treatment of cartilage damage. Poly(ethylene glycol) diacrylate (PEGDA) scaffold. A review.Ital J Anat Embryol. 2013;118(2):204-10. Ital J Anat Embryol. 2013. PMID: 25338410 Review.
-
Polymeric microcarriers for minimally-invasive cell delivery.Front Bioeng Biotechnol. 2023 Jan 26;11:1076179. doi: 10.3389/fbioe.2023.1076179. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36777246 Free PMC article. Review.
Cited by
-
A modular hydrogel bioink containing microsphere-embedded chondrocytes for 3D-printed multiscale composite scaffolds for cartilage repair.iScience. 2023 Jul 11;26(8):107349. doi: 10.1016/j.isci.2023.107349. eCollection 2023 Aug 18. iScience. 2023. PMID: 37539040 Free PMC article.
-
Microcarriers in application for cartilage tissue engineering: Recent progress and challenges.Bioact Mater. 2022 Jan 25;17:81-108. doi: 10.1016/j.bioactmat.2022.01.033. eCollection 2022 Nov. Bioact Mater. 2022. PMID: 35386447 Free PMC article. Review.
-
The Role of Microsphere Structures in Bottom-Up Bone Tissue Engineering.Pharmaceutics. 2023 Jan 18;15(2):321. doi: 10.3390/pharmaceutics15020321. Pharmaceutics. 2023. PMID: 36839645 Free PMC article. Review.
-
Facile Microfluidic Fabrication of Biocompatible Hydrogel Microspheres in a Novel Microfluidic Device.Molecules. 2022 Jun 22;27(13):4013. doi: 10.3390/molecules27134013. Molecules. 2022. PMID: 35807255 Free PMC article.
-
Ginsenosides emerging as both bifunctional drugs and nanocarriers for enhanced antitumor therapies.J Nanobiotechnology. 2021 Oct 15;19(1):322. doi: 10.1186/s12951-021-01062-5. J Nanobiotechnology. 2021. PMID: 34654430 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

