An improved 4'-aminomethyltrioxsalen-based nucleic acid crosslinker for biotinylation of double-stranded DNA or RNA
- PMID: 35515418
- PMCID: PMC9057442
- DOI: 10.1039/d0ra07437c
An improved 4'-aminomethyltrioxsalen-based nucleic acid crosslinker for biotinylation of double-stranded DNA or RNA
Abstract
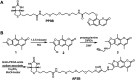
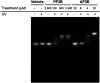
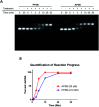
Nucleic acid crosslinkers that covalently join complementary strands of DNA/RNA have applications in both pharmaceuticals and as biochemical probes. Psoralen is a popular crosslinking moiety that reacts with double stranded DNA and RNA upon exposure to longwave UV light. The commercially available compound EZ-link psoralen-PEG3-biotin has been used in numerous studies to crosslink DNA and double-stranded RNA for genome-wide investigations. Here we present a new probe, AP3B, which uses the psoralen derivative, 4'-aminomethyltrioxsalen, to crosslink and biotinylate nucleic acids. We show that AP3B is 4 to 5 times more effective at labeling DNA in cells and produces a comparable number of crosslinks with over 100 times less compound and less exposure to UV light in vitro than EZ-link psoralen-PEG3-biotin.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
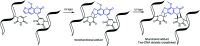
Similar articles
-
Heterogeneous nuclear RNA double-stranded regions probed in living HeLa cells by crosslinking with the psoralen derivative aminomethyltrioxsalen.Proc Natl Acad Sci U S A. 1979 Feb;76(2):755-9. doi: 10.1073/pnas.76.2.755. Proc Natl Acad Sci U S A. 1979. PMID: 284397 Free PMC article.
-
Analysis of RNA secondary structure by photochemical reversal of psoralen crosslinks.Nucleic Acids Res. 1979 Oct 10;7(3):689-703. doi: 10.1093/nar/7.3.689. Nucleic Acids Res. 1979. PMID: 116192 Free PMC article.
-
Accurate measurement of psoralen-crosslinked DNA: direct biochemical measurements and indirect measurement by hybridization.Arch Biochem Biophys. 1988 Nov 1;266(2):351-68. doi: 10.1016/0003-9861(88)90267-6. Arch Biochem Biophys. 1988. PMID: 3142359
-
Electrophoretic and chromatographic separation methods used to reveal interstrand crosslinking of nucleic acids.J Chromatogr. 1993 Aug 25;618(1-2):277-88. doi: 10.1016/0378-4347(93)80038-6. J Chromatogr. 1993. PMID: 8227260 Review.
-
Fundamentals of the psoralen-based Helinx technology for inactivation of infectious pathogens and leukocytes in platelets and plasma.Semin Hematol. 2001 Oct;38(4 Suppl 11):4-11. doi: 10.1016/s0037-1963(01)90118-0. Semin Hematol. 2001. PMID: 11727280 Review.
Cited by
-
Chemical RNA Cross-Linking: Mechanisms, Computational Analysis, and Biological Applications.JACS Au. 2023 Jan 16;3(2):316-332. doi: 10.1021/jacsau.2c00625. eCollection 2023 Feb 27. JACS Au. 2023. PMID: 36873678 Free PMC article. Review.
-
Nanoscale photobiotinylation, pulldown and sequencing of region-specific DNA from intact cells.bioRxiv [Preprint]. 2023 Dec 6:2023.12.04.569951. doi: 10.1101/2023.12.04.569951. bioRxiv. 2023. PMID: 38105945 Free PMC article. Preprint.
-
Repair of genomic interstrand crosslinks.DNA Repair (Amst). 2024 Sep;141:103739. doi: 10.1016/j.dnarep.2024.103739. Epub 2024 Jul 30. DNA Repair (Amst). 2024. PMID: 39106540 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

