Structural properties of the chelating agent 2,6-bis(1-(3-hydroxypropyl)-1,2,3-triazol-4-yl)pyridine: a combined XRD and DFT structural study
- PMID: 35515445
- PMCID: PMC9054079
- DOI: 10.1039/d0ra04142d
Structural properties of the chelating agent 2,6-bis(1-(3-hydroxypropyl)-1,2,3-triazol-4-yl)pyridine: a combined XRD and DFT structural study
Abstract
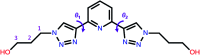
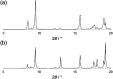
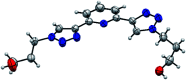
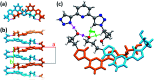
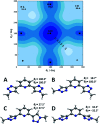
The conformational isomerism of the chelating agent 2,6-bis(1-(3-hydroxypropyl)-1,2,3-triazol-4-yl)pyridine (PTD), exploited in fuel reprocessing in spent nuclear waste, has been studied by single crystal X-ray diffraction analysis in combination with an extensive DFT conformational investigation. In the solid-state, the elucidated crystal structure (i.e., not yet published) shows that by thermal treatment (DSC) no other phases are observed upon crystallization from the melt, indicating that the conformation observed by X-ray data is rather stable. Mapping of intermolecular and intramolecular noncovalent interactions has been used to elucidate the unusual arrangement of the asymmetric unit. Considerations relating to the stability of different conformational isomers in aqueous and non-aqueous solutions are also presented. The accurate structural description reported here might open various research topics such as the potential of PTD to act as an outer sphere ligand in the formation of second sphere coordination complexes and their interconversion by mechanochemical means.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Poinssot Ch. Bourg S. Ouvrier N. Combernoux N. Rostaing C. Vargas-Gonzalez M. Bruno J. Energy. 2014;69:199–211. doi: 10.1016/j.energy.2014.02.069. - DOI
-
- Hill C., in Ion exchange and solvent extraction. A series of advances, ed. B. A. Moyer, CRC Press, Boca Raton, FL, 2019, pp.119–193
-
- Modolo G., Geist A. and Miguirditchian M., in Reprocessing and recycling of spent nuclear fuel, ed. R. Taylor, Woodhead Publishing, Oxford, UK, 2015, pp. 245–287
-
- Mathur J. N. Murali M. S. Nash K. L. Solvent Extr. Ion Exch. 2001;19:357–390. doi: 10.1081/SEI-100103276. - DOI
-
- Bourg S. Hill C. Caravaca C. Rhodes C. Ekberg C. Taylor R. Geist A. Modolo G. Cassayre L. Malmbeck R. Harrison M. de Angelis G. Espartero A. Bouvet S. Ouvrier N. Nucl. Eng. Des. 2011;241:3427–3435. doi: 10.1016/j.nucengdes.2011.03.011. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

