Aqueous solution photocatalytic synthesis of p-anisaldehyde by using graphite-like carbon nitride photocatalysts obtained via the hard-templating route
- PMID: 35515447
- PMCID: PMC9054040
- DOI: 10.1039/d0ra02746d
Aqueous solution photocatalytic synthesis of p-anisaldehyde by using graphite-like carbon nitride photocatalysts obtained via the hard-templating route
Abstract
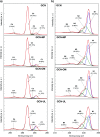
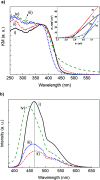
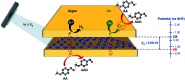
Graphite-like carbon nitride (GCN)-based materials were developed via the hard-templating route, using dicyandiamide as the GCN precursor and silica templates. That resulted in urchin-like GCN (GCN-UL), 3D ordered macroporous GCN (GCN-OM) and mesoporous GCN (GCN-MP). The introduction of silica templates during GCN synthesis produced physical defects on its surface, as confirmed by SEM analysis, increasing their specific surface area. A high amount of nitrogen vacancies is present in modified catalysts (revealed by XPS measurements), which can be related to an increase in the reactive sites available to catalyse redox reactions. The textural and morphological modifications induced in GCN an enhanced light absorption capacity and reduced electron/hole recombination rate, contributing to its improved photocatalytic performance. In the photocatalytic conversion of p-anisyl alcohol to p-anisaldehyde in deoxygenated aqueous solutions under UV-LED irradiation, the GCN-UL was the best photocatalyst reaching 60% yield at 64% conversion for p-anisaldehyde production after 240 min of reaction. Under oxygenated conditions (air), the process efficiency was increased to 79% yield at 92% conversion only after 90 min reaction. The GCN-based photocatalyst kept its performance when using visible-LED radiation under air atmosphere. Trapping of photogenerated holes and radicals by selective scavengers showed that under deoxygenated conditions, holes played the primary role in the p-anisaldehyde synthesis. Under oxygenated conditions, the process is governed by the effect of reactive oxygen species, namely superoxide radicals, with a significant contribution from holes.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Fahlbusch K.-G., Hammerschmidt F.-J., Panten J., Pickenhagen W., Schatkowski D., Bauer K., Garbe D. and Surburg H., in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2012, pp. 74–198
-
- Mukhopadhyay A. K., Derivatives of Para-Cresol, in Industrial Chemical Cresols and Downstream Derivatives, M. Dekker, Taylor & Francis, New York, 2004, pp. 63–96
-
- Friedmann D. Hakki A. Kim H. Choi W. Bahnemann D. Green Chem. 2016;18:5391–5411. doi: 10.1039/C6GC01582D. - DOI
-
- Nakata K. Fujishima A. J. Photochem. Photobiol., C. 2012;13:169–189. doi: 10.1016/j.jphotochemrev.2012.06.001. - DOI
-
- Svoboda L. Praus P. Lima M. J. Sampaio M. J. Matýsek D. Ritz M. Dvorský R. Faria J. L. Silva C. G. Mater. Res. Bull. 2018;100:322–332. doi: 10.1016/j.materresbull.2017.12.049. - DOI
LinkOut - more resources
Full Text Sources

