Oxidative radical coupling of hydroquinones and thiols using chromic acid: one-pot synthesis of quinonyl alkyl/aryl thioethers
- PMID: 35515459
- PMCID: PMC9054077
- DOI: 10.1039/d0ra01519a
Oxidative radical coupling of hydroquinones and thiols using chromic acid: one-pot synthesis of quinonyl alkyl/aryl thioethers
Abstract
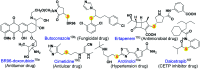
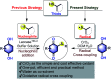
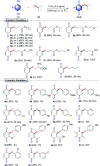
An efficient, simple and practical protocol for one-pot sequential oxidative radical C-H/S-H cross-coupling of thiols with hydroquinones (HQs) and oxidation leading to the formation of quinonyl alkyl/aryl thioethers using H2CrO4 was developed. This cross-coupling of thiyl and aryl radicals offers mono thioethers in good to moderate yield and works well with a wide variety of thiols. Similarly, this method works well for coupling of 2-amino thiophenol and HQs to form phenothiazine-3-ones 5a-c. C-S bond formation via thioether synthesis was observed using a chromium reagent for the first time. Theoretical studies on the pharmacokinetic properties of compounds 5a-c revealed that due to drug-like properties, compound 5b strongly binds with Alzheimer's disease (AD) associated AChE target sites.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
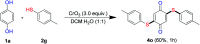

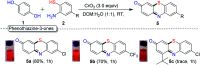

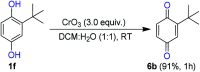
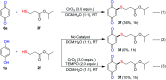
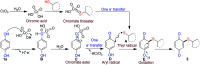
References
-
- Kraus G. A. Mengwasser J. Molecules. 2009;14:2857–2861. doi: 10.3390/molecules14082857. - DOI - PMC - PubMed
- Wendlandt A. E. Stahl S. S. Angew. Chem., Int. Ed. 2015;54:14638–14658. doi: 10.1002/anie.201505017. - DOI - PMC - PubMed
- Hosamani B. Ribeiro M. F. da Silva Junior E. N. Namboothiri I. N. N. Org. Biomol. Chem. 2016;14:6913–6931. doi: 10.1039/C6OB01119E. - DOI - PubMed
- Song C. Dong X. Yi H. Chiang C. W. Lei A. ACS Catal. 2018;8:2195–2199. doi: 10.1021/acscatal.7b04434. - DOI
-
- Dandawate P. R. Vyas A. C. Padhye S. B. Singh M. W. Baruah J. B. Mini-Rev. Med. Chem. 2010;10:436–454. doi: 10.2174/138955710791330909. - DOI - PubMed
- Hadden M. K. Hill S. A. Davenport J. Matts R. L. Blagg B. S. J. Bioorg. Med. Chem. 2009;17:634–640. doi: 10.1016/j.bmc.2008.11.064. - DOI - PMC - PubMed
-
- Madeo J. Zubair A. Marianne F. SpringerPlus. 2013;2(139):1–8. - PMC - PubMed
- Park S. Yun E. Hwanf I. H. Yoon S. Kim D. E. Na M. Song G. Y. Oh S. Mar. Drugs. 2014;12:3231–3244. doi: 10.3390/md12063231. - DOI - PMC - PubMed
- Arai M. Kawachi T. Sato H. Setiawan A. Kobayashi M. Bioorg. Med. Chem. Lett. 2014;24:3155–3157. doi: 10.1016/j.bmcl.2014.04.116. - DOI - PubMed
- Bolton J. L. Dunlap T. Chem. Res. Toxicol. 2017;30:13–37. - PMC - PubMed
- Garcia P. A. Hernández A. P. Feliciano A. S. Castro M. A. Mar. Drugs. 2018;16(292):1–51.
-
- Litchfield V. J. Smith R. B. Franklin A. M. Davis J. Synth. Commun. 2008;38:3447–3455. doi: 10.1080/00397910802154261. - DOI
- Er S. Suh C. Marshaka M. P. Guzik A. A. Chem. Sci. 2015;6:885–893. doi: 10.1039/C4SC03030C. - DOI - PMC - PubMed
- Son E. J. Kim J. H. Kim K. Park C. B. J. Mater. Chem. A. 2016;4:11179–11202. doi: 10.1039/C6TA03123D. - DOI
- Ding Y. Li Y. Yu G. Chem. 2016;1:790–801. doi: 10.1016/j.chempr.2016.09.004. - DOI
- Taran O. Front. Chem. 2017;5(49):1–13. - PMC - PubMed
-
- Sladic D. Gasic M. J. Molecules. 2006;11(1):1–33. doi: 10.3390/11010001. - DOI - PMC - PubMed
- Najjar N. E. Muhtasib H. G. Ketola R. A. Vuorela P. Urtti A. Vuorela H. Phytochem. Rev. 2011;10:353–370. doi: 10.1007/s11101-011-9209-1. - DOI
- Guin P. S. Das S. Mandal P. C. Int. J. Electrochem. 2011;816202:1–22.
LinkOut - more resources
Full Text Sources

