One-pot construction of Cu and O co-doped porous g-C3N4 with enhanced photocatalytic performance towards the degradation of levofloxacin
- PMID: 35515531
- PMCID: PMC9066014
- DOI: 10.1039/c9ra02411e
One-pot construction of Cu and O co-doped porous g-C3N4 with enhanced photocatalytic performance towards the degradation of levofloxacin
Abstract
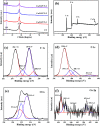
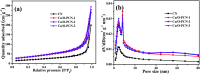
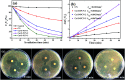
Low visible light response and rapid recombination of photogeneration charge carriers have always been the main factors limiting the advanced application of g-C3N4 (CN). Element doping has been confirmed to be an efficient method to improve the photocatalytic performance of CN. Here, a series of Cu and O co-doped porous g-C3N4 (Cu/O-PCN) nanomaterials were successfully fabricated by a facile one-pot thermal polymerization approach for the first time. Compared to pure CN, the resulting Cu/O-PCN exhibited remarkably enhanced visible-light-driven photocatalytic activity towards levofloxacin (LEVO) degradation. The optimized sample of 0.5% Cu doped (Cu/O-PCN-3) presented the highest degradation rate constant of 0.0676 min-1, which was about 6.2 times higher than that of CN. Furthermore, a substantial decrease in the residual toxicity against E. coli was observed after photocatalytic degradation treatment. The superior photocatalytic performance of Cu/O-PCN was mainly attributed to the synergistic advantages of stronger visible light response, larger specific surface area, and the more effective separation and transfer of photogenerated charge carriers. Moreover, according to the trapping experiments, ·O2 - and h+ were the major oxygen active species in the photocatalytic degradation process. Finally, the possible enhanced photocatalytic mechanism over Cu/O-PCN was proposed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Construction of Pt@BiFeO3 Xerogel-Supported O-g-C3N4 Heterojunction System for Enhanced Visible-Light Activity towards Photocatalytic Degradation of Rhodamine B.Gels. 2023 Jun 8;9(6):471. doi: 10.3390/gels9060471. Gels. 2023. PMID: 37367142 Free PMC article.
-
Ultrathin Ag2WO4-coated P-doped g-C3N4 nanosheets with remarkable photocatalytic performance for indomethacin degradation.J Hazard Mater. 2020 Jun 15;392:122355. doi: 10.1016/j.jhazmat.2020.122355. Epub 2020 Feb 19. J Hazard Mater. 2020. PMID: 32105960
-
One-step preparation of sulfur-doped porous g-C3N4 for enhanced visible light photocatalytic performance.Dalton Trans. 2020 Jun 23;49(24):8041-8050. doi: 10.1039/d0dt00299b. Dalton Trans. 2020. PMID: 32525155
-
Supramolecular self-assembly synthesis of noble-metal-free (C, Ce) co-doped g-C3N4 with porous structure for highly efficient photocatalytic degradation of organic pollutants.J Hazard Mater. 2020 Jan 15;382:121027. doi: 10.1016/j.jhazmat.2019.121027. Epub 2019 Aug 15. J Hazard Mater. 2020. PMID: 31446346
-
Template-mediated copper doped porous g-C3N4 for efficient photodegradation of antibiotic contaminants.Chemosphere. 2022 Apr;293:133607. doi: 10.1016/j.chemosphere.2022.133607. Epub 2022 Jan 12. Chemosphere. 2022. PMID: 35032511
Cited by
-
Effect of Copper-Modification of g-C3N4 on the Visible-Light-Driven Photocatalytic Oxidation of Nitrophenols.Molecules. 2023 Nov 27;28(23):7810. doi: 10.3390/molecules28237810. Molecules. 2023. PMID: 38067540 Free PMC article.
-
Recent progresses in synthesis and modification of g-C3N4 for improving visible-light-driven photocatalytic degradation of antibiotics.Water Sci Technol. 2024 Jun;89(11):3047-3078. doi: 10.2166/wst.2024.166. Epub 2024 May 23. Water Sci Technol. 2024. PMID: 38877630 Review.
-
In situ formation of 2-thiobarbituric acid incorporated g-C3N4 for enhanced visible-light-driven photocatalytic performance.RSC Adv. 2021 Jun 14;11(34):21084-21096. doi: 10.1039/d1ra02121d. eCollection 2021 Jun 9. RSC Adv. 2021. PMID: 35479385 Free PMC article.
-
Enhancing the photocatalytic efficiency of g-C3N4 for ciprofloxacin degradation using Tetrakis (acetonitrile) copper(I) hexafluorophosphate as a highly effective cocatalyst.Heliyon. 2024 Aug 15;10(16):e35829. doi: 10.1016/j.heliyon.2024.e35829. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39253175 Free PMC article.
References
-
- Lillenberg M. Yurchenko S. Kipper K. Herodes K. Pihl V. Lohmus R. Ivask M. Kuu A. Kutti S. Litvin S. V. Nei L. Int. J. Environ. Sci. Technol. 2010;7:307–312. doi: 10.1007/BF03326140. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

