New molecular architectures containing low-valent cluster centres with di- and trimetalated 2-vinylpyrazine ligands: synthesis and molecular structures of Ru5(CO)15(μ5-C4H2N2CH[double bond, length as m-dash]CH)(μ-H)2 and Ru8(CO)24(μ7-C4H2N2CH[double bond, length as m-dash]C)(μ-H)3
- PMID: 35515538
- PMCID: PMC9066017
- DOI: 10.1039/c9ra03841h
New molecular architectures containing low-valent cluster centres with di- and trimetalated 2-vinylpyrazine ligands: synthesis and molecular structures of Ru5(CO)15(μ5-C4H2N2CH[double bond, length as m-dash]CH)(μ-H)2 and Ru8(CO)24(μ7-C4H2N2CH[double bond, length as m-dash]C)(μ-H)3
Abstract
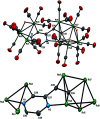
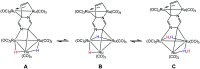
Reaction of 2-vinylpyrazine with Ru3(CO)12 results in multiple C-H bond activations to afford penta- and octa-ruthenium clusters, Ru5(CO)15(μ5-C4H2N2CH[double bond, length as m-dash]CH)(μ-H)2 (2) and Ru8(CO)24(μ7-C4H2N2CH[double bond, length as m-dash]C)(μ-H)3 (3), in which a Ru3 sub-unit is linked to Ru2 and Ru5 centres via di- and tri-metalated 2-vinylpyrazine ligands, exhibiting novel coordination modes including the loss of ring aromaticity in 2. The bonding of 2 and the mechanism for the fluxional behaviour of the hydrides have been examined by electronic structure calculations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

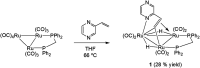




Similar articles
-
Reactions of triosmium and triruthenium clusters with 2-ethynylpyridine: new modes for alkyne C-C bond coupling and C-H bond activation.RSC Adv. 2020 Aug 19;10(51):30671-30682. doi: 10.1039/d0ra05393g. eCollection 2020 Aug 17. RSC Adv. 2020. PMID: 35516016 Free PMC article.
-
Synthesis, Structures, and Transformations of Bridging and Terminally-Coordinated Trimethylammonioalkenyl Ligands in Zwitterionic Pentaruthenium Carbido Carbonyl Complexes.Inorg Chem. 2021 Mar 15;60(6):3781-3793. doi: 10.1021/acs.inorgchem.0c03541. Epub 2021 Feb 22. Inorg Chem. 2021. PMID: 33616385
-
Reactivity of the zwitterionic ligand EtNHC(S)Ph2P[double bond, length as m-dash]NPPh2C(S)NEt towards [Ru3(CO)12]. Sulfur transfer and ligand fragmentation leading to the methideylamide [-N(Et)-CH(R)-] micro3-bridging moiety.Dalton Trans. 2009 Jan 21;(3):544-9. doi: 10.1039/b812050a. Epub 2008 Nov 12. Dalton Trans. 2009. PMID: 19122912
-
Hydrogenating an organometallic carbon chain: buten-yn-diyl (CH[double bond, length as m-dash]CHC[triple bond, length as m-dash]C) as a missing link.Dalton Trans. 2019 Nov 12;48(44):16534-16554. doi: 10.1039/c9dt03229k. Dalton Trans. 2019. PMID: 31576871
-
Equilibria and mesomerism/valence tautomerism of group 4 metallocene complexes.Chem Soc Rev. 2020 Apr 7;49(7):2119-2139. doi: 10.1039/c9cs00637k. Chem Soc Rev. 2020. PMID: 32186300 Review.
Cited by
-
Reactions of triosmium and triruthenium clusters with 2-ethynylpyridine: new modes for alkyne C-C bond coupling and C-H bond activation.RSC Adv. 2020 Aug 19;10(51):30671-30682. doi: 10.1039/d0ra05393g. eCollection 2020 Aug 17. RSC Adv. 2020. PMID: 35516016 Free PMC article.
References
-
- Oi S. Tanaka Y. Inoue Y. Organometallics. 2006;25:4773–4778. doi: 10.1021/om060561k. - DOI
-
- Wong-Foy A. G. Henling L. M. Day M. Labinger J. A. Bercaw J. E. J. Mol. Catal. A: Chem. 2002;189:3–16. doi: 10.1016/S1381-1169(02)00193-0. - DOI
-
- Navarro J. Sola E. Martín M. Dobrinovitch I. T. Lahoz F. J. Oro L. A. Organometallics. 2004;23:1908–1917. doi: 10.1021/om049953m. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

